A water heater of power $2000 W$ is used to heat water. The specific heat capacity of water is $4200 J$ $kg ^{-1} K ^{-1}$ .The efficiency of heater is $70 \%$ .Time required to heat $2 kg$ of water from $10^{\circ} C$ to $60^{\circ} C$ is___$ s$
(Assume that the specific heat capacity of water remains constant over the temperature range of the water)
A water heater of power $2000 W$ is used to heat water. The specific heat capacity of water is $4200 J$ $kg ^{-1} K ^{-1}$ .The efficiency of heater is $70 \%$ .Time required to heat $2 kg$ of water from $10^{\circ} C$ to $60^{\circ} C$ is___$ s$
(Assume that the specific heat capacity of water remains constant over the temperature range of the water)
Show Hint
Correct Answer: 300
Solution and Explanation
Top Questions on specific heat capacity
Match List-I with List-II.
List-I List-II (A) Heat capacity of body (I) \( J\,kg^{-1} \) (B) Specific heat capacity of body (II) \( J\,K^{-1} \) (C) Latent heat (III) \( J\,kg^{-1}K^{-1} \) (D) Thermal conductivity (IV) \( J\,m^{-1}K^{-1}s^{-1} \) - JEE Main - 2025
- Physics
- specific heat capacity
- Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
- KEAM - 2025
- Physics
- specific heat capacity
- If \( \frac{C_p}{C_v} \) is unity for a process, \( PV^{\gamma} = \text{constant} \), then the process is:
- KEAM - 2025
- Physics
- specific heat capacity
- Specific heat capacity of diatomic gas at constant volume:
- KEAM - 2025
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
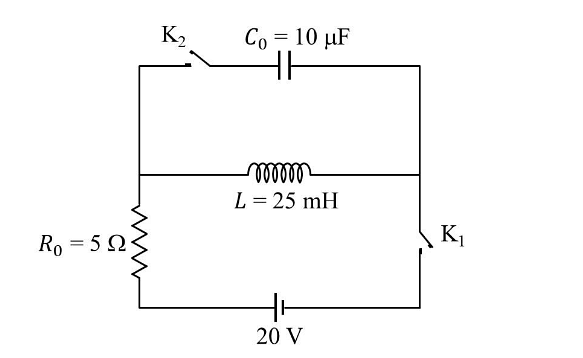
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
Questions Asked in JEE Main exam
- Let the circle \(x^2+y^2=4\) intersect the \(x\)-axis at points \(A(a,0)\) and \(B(b,0)\). Let \(P(2\cos\alpha,2\sin\alpha)\), \(0<\alpha<\frac{\pi}{2}\), and \(Q(2\cos\beta,2\sin\beta)\) be two points on the circle such that \((\alpha-\beta)=\frac{\pi}{2}\). Then the point of intersection of lines \(AQ\) and \(BP\) lies on:
- JEE Main - 2026
- Circles
- The moment of inertia of a square loop made of four uniform solid cylinders, each having radius R and length L (\(R \le L\)) about an axis passing through the mid points of opposite sides, is (Take the mass of the entire loop as M) :
- JEE Main - 2026
- Kinematics
- When a light of a given wavelength falls on a metallic surface the stopping potential for photoelectrons is \(3.2\ \text{V}\). If a second light having wavelength twice of the first light is used, the stopping potential drops to \(0.7\ \text{V}\). The wavelength of the first light is ________ m.
- JEE Main - 2026
- Photoelectric Effect
- An infinitely long straight wire carrying current $I$ is bent in a planar shape as shown in the diagram. The radius of the circular part is $r$. The magnetic field at the centre $O$ of the circular loop is :

- JEE Main - 2026
- Current electricity
- Identify the correct statements: The presence of –NO\(_2\) group in benzene ring A. activates the ring towards electrophilic substitutions. B. deactivates the ring towards electrophilic substitutions. C. activates the ring towards nucleophilic substitutions. D. deactivates the ring towards nucleophilic substitutions. Choose the correct answer from the options given below:
- JEE Main - 2026
- General Chemistry
Concepts Used:
Thermodynamics
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
Important Terms
System
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable.
There are three types of systems:
- Isolated System – An isolated system cannot exchange both energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerators and compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic process that have their unique properties, and they are:
- Adiabatic Process – A process in which no heat transfer takes place.
- Isochoric Process – A thermodynamic process taking place at constant volume is known as the isochoric process.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Laws of Thermodynamics
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
First Law of Thermodynamics
The First law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing three kinds of transfer of energy, as heat, as thermodynamic work, and as energy associated with matter transfer, and relating them to a function of a body's state, called internal energy.
Second Law of Thermodynamics
The Second law of thermodynamics is a physical law of thermodynamics about heat and loss in its conversion.
Third Law of Thermodynamics
Third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.