Question:
Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
Show Hint
The product of uncertainty in position and uncertainty in velocity has units of momentum, which are \(m \cdot s^{-1}\).
Updated On: Apr 28, 2025
- \(m\cdot s\)
- \(m^2\cdot s^{-1}\)
- \(m\cdot s^{-1}\)
- \(m^2\cdot s^2\)
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
The Heisenberg uncertainty principle relates the uncertainty in position (\(\Delta x\)) and the uncertainty in momentum (\(\Delta p\)), which can be expressed as: \[ \Delta x \cdot \Delta p \geq \frac{\hbar}{2} \] Where: - \(\hbar\) is the reduced Planck constant. Momentum is defined as \(p = m \cdot v\), where \(m\) is mass and \(v\) is velocity. Therefore, the uncertainty in momentum is: \[ \Delta p = m \cdot \Delta v \] Substituting into the uncertainty principle: \[ \Delta x \cdot m \cdot \Delta v \geq \frac{\hbar}{2} \] This shows that the product of uncertainty in position and uncertainty in velocity has units of: \[ \Delta x \cdot \Delta v \sim m \cdot s^{-1} \] Thus, the correct unit for the product of \(\Delta x\) and \(\Delta v\) is \(m \cdot s^{-1}\).
Was this answer helpful?
0
1
Top Questions on specific heat capacity
Match List-I with List-II.
List-I List-II (A) Heat capacity of body (I) \( J\,kg^{-1} \) (B) Specific heat capacity of body (II) \( J\,K^{-1} \) (C) Latent heat (III) \( J\,kg^{-1}K^{-1} \) (D) Thermal conductivity (IV) \( J\,m^{-1}K^{-1}s^{-1} \) - JEE Main - 2025
- Physics
- specific heat capacity
- If \( \frac{C_p}{C_v} \) is unity for a process, \( PV^{\gamma} = \text{constant} \), then the process is:
- KEAM - 2025
- Physics
- specific heat capacity
- Specific heat capacity of diatomic gas at constant volume:
- KEAM - 2025
- Physics
- specific heat capacity
In the given cycle ABCDA, the heat required for an ideal monoatomic gas will be:

- BITSAT - 2024
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
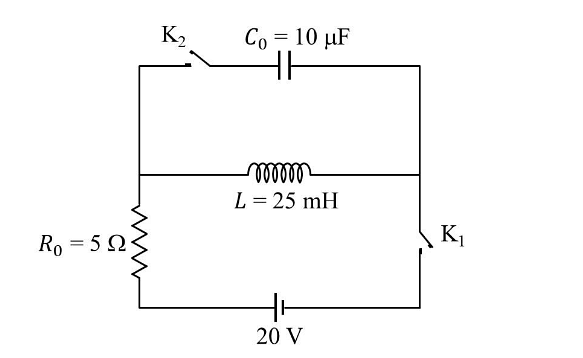
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
View More Questions
Questions Asked in KEAM exam
- Which among the following has the highest molar elevation constant?
- KEAM - 2025
- Colligative Properties
- The formula of Ammonium phosphomolybdate is
- KEAM - 2025
- coordination compounds
- Which is a Lewis acid?
- KEAM - 2025
- Acids and Bases
- Hardness of water is estimated by titration with
- KEAM - 2025
- Solutions
- Which of the following gases has the lowest solubility in water at 298 K?
- KEAM - 2025
- Solutions
View More Questions