Question:
Specific heat capacity of diatomic gas at constant volume:
Specific heat capacity of diatomic gas at constant volume:
Show Hint
The specific heat capacity of a diatomic gas at constant volume is \(\frac{5}{2} R\), which is higher than that of monatomic gases, due to additional rotational and vibrational modes.
Updated On: Apr 25, 2025
- \(\frac{5}{2} R\)
- \(R\)
- \(\frac{3}{2} R\)
- \(\frac{7}{2} R\)
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
For an ideal diatomic gas, the specific heat capacity at constant volume (\(C_V\)) is given by: \[ C_V = \frac{5}{2} R \] Where: - \(R\) is the universal gas constant. This result is derived from the degrees of freedom of the diatomic gas molecules. A diatomic gas has translational, rotational, and vibrational degrees of freedom, and for an ideal gas, the specific heat at constant volume is based on the energy associated with these degrees of freedom. Thus, the specific heat capacity of a diatomic gas at constant volume is \(\frac{5}{2} R\).
Was this answer helpful?
0
0
Top Questions on specific heat capacity
Match List-I with List-II.
List-I List-II (A) Heat capacity of body (I) \( J\,kg^{-1} \) (B) Specific heat capacity of body (II) \( J\,K^{-1} \) (C) Latent heat (III) \( J\,kg^{-1}K^{-1} \) (D) Thermal conductivity (IV) \( J\,m^{-1}K^{-1}s^{-1} \) - JEE Main - 2025
- Physics
- specific heat capacity
- Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
- KEAM - 2025
- Physics
- specific heat capacity
- If \( \frac{C_p}{C_v} \) is unity for a process, \( PV^{\gamma} = \text{constant} \), then the process is:
- KEAM - 2025
- Physics
- specific heat capacity
In the given cycle ABCDA, the heat required for an ideal monoatomic gas will be:

- BITSAT - 2024
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
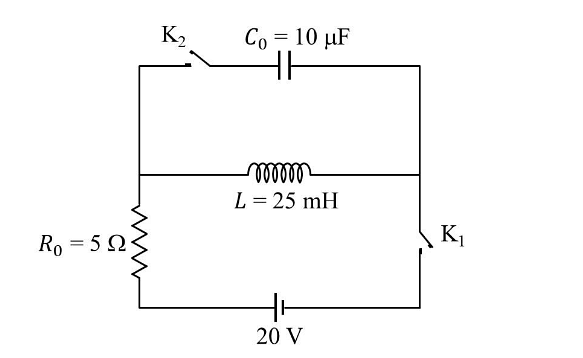
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
View More Questions
Questions Asked in KEAM exam
- Which among the following has the highest molar elevation constant?
- KEAM - 2025
- Colligative Properties
- The formula of Ammonium phosphomolybdate is
- KEAM - 2025
- coordination compounds
- Which is a Lewis acid?
- KEAM - 2025
- Acids and Bases
- Hardness of water is estimated by titration with
- KEAM - 2025
- Solutions
- Which of the following gases has the lowest solubility in water at 298 K?
- KEAM - 2025
- Solutions
View More Questions