The value of $\frac{1}{1 ! 50 !}+\frac{1}{3 ! 48 !}+\frac{1}{5 ! 46 !}+\ldots +\frac{1}{49 ! 2 !}+\frac{1}{51 ! 1 !}$ is :
- $\frac{2^{51}}{50 !}$
- $\frac{2^{50}}{50 !}$
- $\frac{2^{51}}{51 !}$
- $\frac{2^{50}}{51 !}$
The Correct Option is D
Approach Solution - 1
\(∑_{r=1}^{26} \frac{1}{(2r−1)!(51−(2r−1))!} \)
\(= ∑_{r=1}^{26} ^{51}C_{(2r−1)} \frac{1}{51!}\)
\(=\frac{1}{51!} {^{51}C_1 + ^{51}C_3 +….+ ^{51}C_{51}}= \frac{1}{51!} (2^{50})\)
\(= (\frac{2^{50}}{51!} )\)
Therefore, The correct answer is option (D): \(\frac{2^{50}}{51!} \)
Approach Solution -2
\[ \sum_{r=1}^{26} \frac{1}{(2r-1)! (51-(2r-1))!} = \sum_{r=1}^{26} \binom{51}{2r-1} \frac{1}{51!} \] \[ = \frac{1}{51!} \sum_{r=1}^{26} \binom{51}{2r-1} = \frac{1}{51!} (2^{50}) \]
Top Questions on Definite Integral
- Evaluate the definite integral: \( \int_{-2}^{2} |x^2 - x - 2| \, dx \)
- MHT CET - 2025
- Mathematics
- Definite Integral
- For \( x \in \left( -\frac{\pi}{2}, \frac{\pi}{2} \right) \), if\[y(x) = \int \frac{\csc x + \sin x}{\csc x \sec x + \tan x \sin^2 x} \, dx\]and\[\lim_{x \to -\frac{\pi}{2}} y(x) = 0\]then \( y\left(\frac{\pi}{4}\right) \) is equal to
- JEE Main - 2024
- Mathematics
- Definite Integral
- The value of the integral \[ \int_{-1}^{2} \log_e \left( x + \sqrt{x^2 + 1} \right) \, dx \] is:
- JEE Main - 2024
- Mathematics
- Definite Integral
The value \( 9 \int_{0}^{9} \left\lfloor \frac{10x}{x+1} \right\rfloor \, dx \), where \( \left\lfloor t \right\rfloor \) denotes the greatest integer less than or equal to \( t \), is ________.
- JEE Main - 2024
- Mathematics
- Definite Integral
- The value of \(\lim_{{n \to \infty}} \sum_{{k=1}}^{n} \frac{n^3}{{(n^2 + k^2)(n^2 + 3k^2)}}\) is
- JEE Main - 2024
- Mathematics
- Definite Integral
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- The work functions of two metals ($M_A$ and $M_B$) are in the 1 : 2 ratio. When these metals are exposed to photons of energy 6 eV, the kinetic energy of liberated electrons of $M_A$ : $M_B$ is in the ratio of 2.642 : 1. The work functions (in eV) of $M_A$ and $M_B$ are respectively.
- JEE Main - 2026
- Dual nature of matter
Concepts Used:
Definite Integral
Definite integral is an operation on functions which approximates the sum of the values (of the function) weighted by the length (or measure) of the intervals for which the function takes that value.
Definite integrals - Important Formulae Handbook
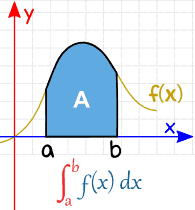
A real valued function being evaluated (integrated) over the closed interval [a, b] is written as :
\(\int_{a}^{b}f(x)dx\)
Definite integrals have a lot of applications. Its main application is that it is used to find out the area under the curve of a function, as shown below: