Question:
The normal magnetic flux passing through a coil changes with time according to the equation $\phi = 6t^2 - 5t + 1$. What is the magnitude of the induced current at $t = 0.253\, s$ and resistance $10 \, \Omega $ ?
The normal magnetic flux passing through a coil changes with time according to the equation $\phi = 6t^2 - 5t + 1$. What is the magnitude of the induced current at $t = 0.253\, s$ and resistance $10 \, \Omega $ ?
Updated On: May 12, 2024
- 1.2 A
- 0.8 A
- 0.6 A
- 0.2 A
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
Here ,
Magnetic flux, $\phi = 6t^2 - 5t + 1$
Resistance, $R = 10\, \Omega$
The induced emf is
$\varepsilon = - \frac{d\phi}{dt} = - \frac{d}{dt} \left(6t^{2} - 5t+ 1\right)=-\left(12t - 5 \right) $
At $t = 0.253 \,s$
$\varepsilon = -(12 \times 0.253 - 5 ) = 1.964 \, V = 2 V$
$\therefore$ Induced current, $ I = \frac{ \varepsilon}{R} = \frac{2}{10} = 0.2 A$
Magnetic flux, $\phi = 6t^2 - 5t + 1$
Resistance, $R = 10\, \Omega$
The induced emf is
$\varepsilon = - \frac{d\phi}{dt} = - \frac{d}{dt} \left(6t^{2} - 5t+ 1\right)=-\left(12t - 5 \right) $
At $t = 0.253 \,s$
$\varepsilon = -(12 \times 0.253 - 5 ) = 1.964 \, V = 2 V$
$\therefore$ Induced current, $ I = \frac{ \varepsilon}{R} = \frac{2}{10} = 0.2 A$
Was this answer helpful?
0
0
Top Questions on Faradays laws of induction
- In a coil, the current changes form –2 A to +2A in 0.2 s and induces an emf of 0.1 V. The self-inductance of the coil is :
- JEE Main - 2024
- Physics
- Faradays laws of induction
- The magnetic flux \(\phi\) (in weber) linked with a closed circuit of resistance \(8 \, \Omega\) varies with time (in seconds) as \(\phi = 5t^2 - 36t + 1\). The induced current in the circuit at \(t = 2 \, \text{s}\) is ______ A.
- JEE Main - 2024
- Physics
- Faradays laws of induction
- A square loop of side 2 cm enters a magnetic field with a constant speed of 2 cm s-1 as shown. The front edge enters the field at t = 0s. Which of the following graph correctly depicts the induced emf in the loop?
( Take clockwise direction positive )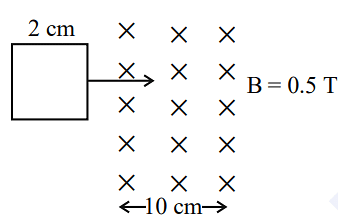
- KCET - 2023
- Physics
- Faradays laws of induction
- A metallic rod of length 1 m held along east-west direction is allowed to fall down freely. Given horizontal component of earth’s magnetic field BH = 3 × 10-5 T. The emf induced in the rod at an instant t = 2s after it is released is ( Take g = 10 ms-2 )
- KCET - 2023
- Physics
- Faradays laws of induction
- The current following through an inductance coil of self inductance 6 mH at different time instants is as shown. The emf induced between t = 20s and t = 40s is nearly
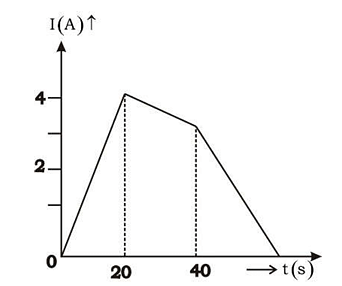
- KCET - 2021
- Physics
- Faradays laws of induction
View More Questions
Questions Asked in COMEDK UGET exam
- Given that the freezing point of benzene is $ 5.48^\circ C $ and its $ K_f $ value is $ 5.12^\circ C/m $, what would be the freezing point of a solution of 20 g of propane in 400 g of benzene?
- COMEDK UGET - 2024
- Colligative Properties
200 ml of an aqueous solution contains 3.6 g of Glucose and 1.2 g of Urea maintained at a temperature equal to 27$^{\circ}$C. What is the Osmotic pressure of the solution in atmosphere units?
Given Data R = 0.082 L atm K$^{-1}$ mol$^{-1}$
Molecular Formula: Glucose = C$_6$H$_{12}$O$_6$, Urea = NH$_2$CONH$_2$- COMEDK UGET - 2024
- Colligative Properties
- An inorganic compound W undergoes the following reactions: $ W + \text{Na}_2\text{CO}_3 \xrightarrow{\text{O}_2 / \text{heat}} X + H^+ \xrightarrow{} Y(s) $ $ Y(aq) + \text{KCl} (aq) \xrightarrow{} Z(s) $ Z appears in the form of orange crystals and is used as an oxidising agent in acid medium. Identify the compound W.
- COMEDK UGET - 2024
- coordination compounds
- A current of 3.0 A is passed through 750 ml of 0.45 M solution of CuSO₄ for 2 hours with a current efficiency of 90\%. If the volume of the solution is assumed to remain constant, what would be the final molarity of CuSO₄ solution?
- COMEDK UGET - 2024
- Solutions
- For a reaction $ 5X + Y \to 3Z $, the rate of formation of Z is $ 2.4 \times 10^{-5} \, \text{mol L}^{-1} \text{s}^{-1} $. Calculate the average rate of disappearance of X.
- COMEDK UGET - 2024
- Stoichiometry and Stoichiometric Calculations
View More Questions
Concepts Used:
Faradays Laws of Induction
There are two laws, given by Faraday which explain the phenomena of electromagnetic induction:
Faraday's First Law:
Whenever a conductor is placed in a varying magnetic field, an emf is induced. If the conductor circuit is closed, a current is induced, known as the induced current.
Faraday's Second Law:
The Emf induced inside a coil is equal to the rate of change of associated magnetic flux.
This law can be mathematically written as:
∈\(-N {\triangle \phi \over \triangle t}\)
