Which one of the following reactions indicates the reducing ability of hydrogen peroxide in basic medium?
\(HOCI + H_2O_2 → H_3O+ + CI^– + O_2\)
\(PbS + 4H_2O_2 → PbSO_4 + 4H_2O\)
\(2MnO^-_4, +3H_2O_2 → 2MnO_2, +3O_2, +2H_2O + 2OH^- \)
\(MN^{2+} + H_2O_2 → Mn^{4+}+2OH^-\)
The Correct Option is C
Solution and Explanation
In basic medium \(MnO_4^– \) is reduced to \(MnO_2\), whereas in acidic medium it is reduced to \(Mn^{+2}\).
So, the correct option is (C): \(2MnO^-_4, +3H_2O_2 → 2MnO_2, +3O_2, +2H_2O + 2OH^- \)
Top Questions on Chemical Reactions
- One mole of Cl$_2$(g) was passed into 2 L of cold 2 M KOH solution. After the reaction, the concentrations of Cl$^-$, ClO$^-$ and OH$^-$ are respectively (assume volume remains constant)
- JEE Main - 2026
- Chemistry
- Chemical Reactions
The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
- Example of thermal decomposition reaction are:
- \( \text{2AgCl} \rightarrow \text{2Ag} + \text{Cl}_2 \)
- \( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \)
- \( \text{2H}_2\text{O} \rightarrow \text{2H}_2 + \text{O}_2 \)
- \( \text{2KClO}_3 \rightarrow \text{2KCl} + \text{3O}_2 \)
- CBSE Class X - 2025
- Science
- Chemical Reactions
- In which one of the following situations a chemical reaction does not occur?
- CBSE Class X - 2025
- Science
- Chemical Reactions
- The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- The work functions of two metals ($M_A$ and $M_B$) are in the 1 : 2 ratio. When these metals are exposed to photons of energy 6 eV, the kinetic energy of liberated electrons of $M_A$ : $M_B$ is in the ratio of 2.642 : 1. The work functions (in eV) of $M_A$ and $M_B$ are respectively.
- JEE Main - 2026
- Dual nature of matter
Concepts Used:
Types of Differential Equations
There are various types of Differential Equation, such as:
Ordinary Differential Equations:
Ordinary Differential Equations is an equation that indicates the relation of having one independent variable x, and one dependent variable y, along with some of its other derivatives.
\(F(\frac{dy}{dt},y,t) = 0\)
Partial Differential Equations:
A partial differential equation is a type, in which the equation carries many unknown variables with their partial derivatives.
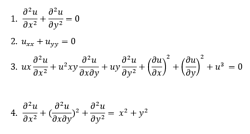
Linear Differential Equations:
It is the linear polynomial equation in which derivatives of different variables exist. Linear Partial Differential Equation derivatives are partial and function is dependent on the variable.
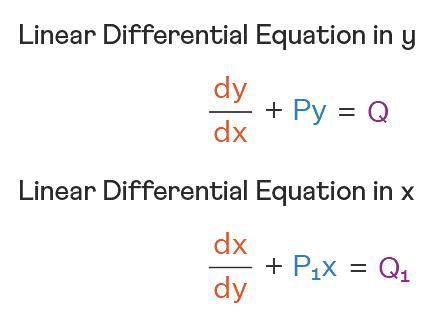
Homogeneous Differential Equations:
When the degree of f(x,y) and g(x,y) is the same, it is known to be a homogeneous differential equation.
\(\frac{dy}{dx} = \frac{a_1x + b_1y + c_1}{a_2x + b_2y + c_2}\)
Read More: Differential Equations