Question:
The major product P formed in the given reaction is: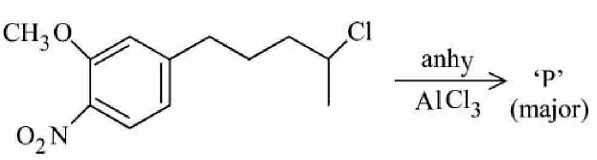
The major product P formed in the given reaction is:

Updated On: Feb 4, 2026
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The given reaction is an electrophilic aromatic substitution. When the compound reacts with \( \text{AlCl}_3 \), a Friedel-Crafts alkylation or acylation typically takes place. The compound contains both a nitro group (\( \text{NO}_2 \)) and a methoxy group (\( \text{OCH}_3 \)) as substituents.
The nitro group is electron-withdrawing and deactivates the aromatic ring towards electrophilic substitution, while the methoxy group is electron-donating and activates the ring. Therefore, the reaction will predominantly occur at the position where the methoxy group is located, as it makes the ring more reactive.
As a result, the major product will be the structure shown in option (1), where substitution occurs at the position activated by the methoxy group.
The nitro group is electron-withdrawing and deactivates the aromatic ring towards electrophilic substitution, while the methoxy group is electron-donating and activates the ring. Therefore, the reaction will predominantly occur at the position where the methoxy group is located, as it makes the ring more reactive.
As a result, the major product will be the structure shown in option (1), where substitution occurs at the position activated by the methoxy group.
Was this answer helpful?
0
3
Top Questions on Chemical Reactions
- One mole of Cl$_2$(g) was passed into 2 L of cold 2 M KOH solution. After the reaction, the concentrations of Cl$^-$, ClO$^-$ and OH$^-$ are respectively (assume volume remains constant)
- JEE Main - 2026
- Chemistry
- Chemical Reactions
The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
- Example of thermal decomposition reaction are:
- \( \text{2AgCl} \rightarrow \text{2Ag} + \text{Cl}_2 \)
- \( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \)
- \( \text{2H}_2\text{O} \rightarrow \text{2H}_2 + \text{O}_2 \)
- \( \text{2KClO}_3 \rightarrow \text{2KCl} + \text{3O}_2 \)
- CBSE Class X - 2025
- Science
- Chemical Reactions
- In which one of the following situations a chemical reaction does not occur?
- CBSE Class X - 2025
- Science
- Chemical Reactions
- The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
View More Questions
Questions Asked in JEE Main exam
- Let $\vec{a}=2\hat{i}-\hat{j}-\hat{k}$, $\vec{b}=\hat{i}+3\hat{j}-\hat{k}$ and $\vec{c}=2\hat{i}+\hat{j}+3\hat{k}$. Let $\vec{v}$ be the vector in the plane of $\vec{a}$ and $\vec{b}$, such that the length of its projection on the vector $\vec{c}$ is $\dfrac{1}{\sqrt{14}}$. Then $|\vec{v}|$ is equal to
- JEE Main - 2026
- Vector Algebra
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- A 20 m long uniform copper wire held horizontally is allowed to fall under the gravity (g = 10 m/s²) through a uniform horizontal magnetic field of 0.5 Gauss perpendicular to the length of the wire. The induced EMF across the wire when it travels a vertical distance of 200 m is_______ mV.}
- JEE Main - 2026
- Thermodynamics
- If the end points of chord of parabola \(y^2 = 12x\) are \((x_1, y_1)\) and \((x_2, y_2)\) and it subtend \(90^\circ\) at the vertex of parabola then \((x_1x_2 - y_1y_2)\) equals :
- JEE Main - 2026
- Probability
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
View More Questions



