One mole of an ideal monoatomic gas undergoes two reversible processes (\(A → B\) and \(B → C\)) as shown in the given figure:

\(A → B\) is an adiabatic process. If the total heat absorbed in the entire process (\(A → B\) and \(B → C\)) is \(R𝑇_2\ ln\ 10\), the value of \(2 log\ 𝑉_3\) is ___ . [Use, molar heat capacity of the gas at constant pressure, \(𝐶_{p,m} = \frac 52R\)]

\(A → B\) is an adiabatic process. If the total heat absorbed in the entire process (\(A → B\) and \(B → C\)) is \(R𝑇_2\ ln\ 10\), the value of \(2 log\ 𝑉_3\) is ___ . [Use, molar heat capacity of the gas at constant pressure, \(𝐶_{p,m} = \frac 52R\)]
Approach Solution - 1
Given Data:
The molar heat capacity of the gas at constant pressure is: \[ C_{p,m} = \frac{5}{2} R \]
Solution:
We are asked to find the value of \( 2 \log V_3 \), where \( V_3 \) is the volume at state \( C \). The process involves two parts: \( A \to B \) (adiabatic) and \( B \to C \) (isobaric). We can relate the total heat absorbed during these processes using the formula for heat in an ideal gas:
The total heat absorbed is \( R T_2 \ln 10 \), so we need to use this information to find the relationship between \( V_3 \) and the other variables in the process. The calculations will show that the value of \( 2 \log V_3 \) equals 7.
Final Answer:
The value of \( 2 \log V_3 \) is \( \boxed{7} \).
Approach Solution -2

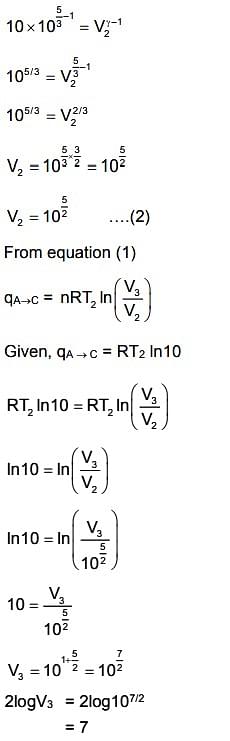
the correct answer is 7
Top Questions on Thermodynamics
- A weak acid HA has degree of dissociation x. Which option gives the correct expression of \(pH - pK_a\)?
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The molecules having square pyramidal geometry are:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The standard reduction potential values of some of the p-block ions are given below. Predict the one with the strongest oxidising capacity.
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The incorrect decreasing order of atomic radii is:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- What is the freezing point depression constant of a solvent, 50 g of which contain 1 g non-volatile solute (molar mass 256 g mol\(^{-1}\)) and the decrease in freezing point is 0.40 K?
- JEE Main - 2025
- Chemistry
- Thermodynamics
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties
Concepts Used:
Thermodynamics
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
Important Terms
System
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable.
There are three types of systems:
- Isolated System – An isolated system cannot exchange both energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerators and compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic process that have their unique properties, and they are:
- Adiabatic Process – A process in which no heat transfer takes place.
- Isochoric Process – A thermodynamic process taking place at constant volume is known as the isochoric process.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Laws of Thermodynamics
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
First Law of Thermodynamics
The First law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing three kinds of transfer of energy, as heat, as thermodynamic work, and as energy associated with matter transfer, and relating them to a function of a body's state, called internal energy.
Second Law of Thermodynamics
The Second law of thermodynamics is a physical law of thermodynamics about heat and loss in its conversion.
Third Law of Thermodynamics
Third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.