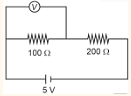
In given circuit, reading of voltmeter is 1 V, then resistance of

- 100 Ω
- 200 Ω
- 200√5 Ω
- 50 Ω
The Correct Option is A
Solution and Explanation
The problem asks for the resistance of a voltmeter, given its reading when connected in parallel across a 100 Ω resistor in a series circuit with a 200 Ω resistor and a 5 V source.
Concept Used:
The solution utilizes the following principles of electric circuits:
1. Ohm's Law: The potential difference (voltage) \( V \) across a resistor is proportional to the current \( I \) flowing through it, given by \( V = IR \).
2. Resistors in Series: The total voltage across components in series is the sum of the individual voltages. The current is the same through all components.
3. Resistors in Parallel: The equivalent resistance \( R_p \) of two resistors \( R_a \) and \( R_b \) connected in parallel is given by:
\[ \frac{1}{R_p} = \frac{1}{R_a} + \frac{1}{R_b} \quad \text{or} \quad R_p = \frac{R_a R_b}{R_a + R_b} \]A voltmeter has its own internal resistance and is always connected in parallel to the component across which the voltage is measured.
Step-by-Step Solution:
Step 1: Identify the given parameters from the circuit diagram.
Source voltage, \( V_s = 5 \, \text{V} \).
First resistor, \( R_1 = 100 \, \Omega \).
Second resistor, \( R_2 = 200 \, \Omega \).
Reading of the voltmeter across \( R_1 \), \( V_m = 1 \, \text{V} \).
Let the resistance of the voltmeter be \( R_V \).
Step 2: Determine the voltage across the 200 Ω resistor.
The voltmeter and the 100 Ω resistor form a parallel combination. This combination is in series with the 200 Ω resistor. The sum of the voltage across the parallel part (\( V_m \)) and the voltage across the 200 Ω resistor (\( V_{200} \)) must equal the source voltage.
\[ V_s = V_m + V_{200} \] \[ 5 \, \text{V} = 1 \, \text{V} + V_{200} \] \[ V_{200} = 5 - 1 = 4 \, \text{V} \]Step 3: Calculate the total current flowing from the source.
This total current \( I \) flows through the 200 Ω resistor. Using Ohm's law for this resistor:
\[ I = \frac{V_{200}}{R_2} = \frac{4 \, \text{V}}{200 \, \Omega} = \frac{1}{50} \, \text{A} = 0.02 \, \text{A} \]Step 4: Calculate the equivalent resistance of the parallel combination.
The voltmeter reading \( V_m = 1 \, \text{V} \) is the potential difference across the parallel combination of the 100 Ω resistor and the voltmeter. Let the equivalent resistance of this parallel part be \( R_p \). Using Ohm's law for this part:
\[ V_m = I \times R_p \] \[ 1 \, \text{V} = (0.02 \, \text{A}) \times R_p \] \[ R_p = \frac{1}{0.02} = 50 \, \Omega \]Step 5: Use the equivalent parallel resistance to find the voltmeter's resistance \( R_V \).
The equivalent resistance \( R_p \) is the result of the parallel combination of \( R_1 = 100 \, \Omega \) and \( R_V \).
\[ R_p = \frac{R_1 \times R_V}{R_1 + R_V} \]Substitute the known values:
\[ 50 = \frac{100 \times R_V}{100 + R_V} \]Final Computation & Result:
Solve the equation for \( R_V \):
\[ 50(100 + R_V) = 100 R_V \] \[ 5000 + 50 R_V = 100 R_V \] \[ 5000 = 100 R_V - 50 R_V \] \[ 5000 = 50 R_V \] \[ R_V = \frac{5000}{50} = 100 \, \Omega \]The resistance of the voltmeter is 100 Ω.
Top Questions on Heat Transfer
- In the steady state of temperature, the flow of heat across the body depends upon its:
A. thermal capacity
B. thermal conductivity
C. temperature difference across its opposite faces
D. thermal resistivity
Choose the CORRECT answer from the options given below:- CUET (PG) - 2025
- Physics
- Heat Transfer
Match the LIST-I with LIST-II
LIST-I LIST-II (Type of Fouling) (Fouling Mechanism) A Precipitation IV Precipitation of dissolved substances... B Freezing III Solidification of Liquid components... C Particulate I Accumulation of fine particles suspended... D Corrosion II Heat transfer surface reacts with ambient... Identify the evaporator

- The difference between the initial and the final temperature divided by the freezing time is known as
- The Nusselt number is related to the Reynolds number in laminar and turbulent flows respectively as:
- CUET (PG) - 2025
- Mechanical Engineering
- Heat Transfer
Questions Asked in JEE Main exam
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Concepts Used:
Specific Heat Capacity
Specific heat of a solid or liquid is the amount of heat that raises the temperature of a unit mass of the solid through 1°C.
Molar Specific Heat:
The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1°C.
Specific Heat at Constant Pressure or Volume:
The volume of solid remains constant when heated through a small range of temperature. This is known as specific heat at a constant volume. It is denoted as CV.
The pressure of solid remains constant when heated through a small range of temperature. This is known as specific heat at constant pressure which can be denoted as CP.