Question:
Ceramides are
Ceramides are
Show Hint
Ceramides are involved in maintaining the structural integrity of cell membranes and play a role in regulating cell apoptosis and differentiation.
Updated On: May 5, 2025
- Protein synthesized by endoplasmic reticulum
- Sphingolipid synthesized by endoplasmic reticulum
- Carbohydrate synthesized by endoplasmic reticulum
- Sphingolipid synthesized by Golgi apparatus
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
Ceramides are a class of sphingolipids formed by the linkage of sphingosine to a fatty acid. They are synthesized in the endoplasmic reticulum and play key roles in cell signaling and the formation of the skin's barrier.
Was this answer helpful?
0
0
Top Questions on Lipids
- A patient presents with xanthomas on the Achilles tendon. Which of the following is the most likely diagnosis?
- A patient with multiple tendon xanthomas is found to have a serum cholesterol level of 398 mg/dL and an LDL level of 220 mg/dL. What is the most likely defect?
- Which of the following has the lowest melting point?
- A child presents with bone pain and hepatosplenomegaly. A trephine biopsy and aspirate show the following finding. Which of the following is the most likely enzyme deficient in this condition?
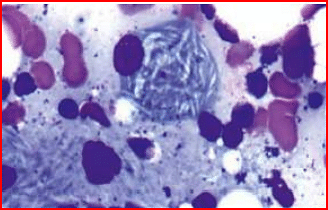
- A child presents with mental retardation, bone pain, and inability to walk. On funduscopic examination, a cherry red spot is seen. There is no organomegaly. What is the most likely diagnosis?
View More Questions
Questions Asked in AP PGECET exam
- The absolute humidity of air at 101.325 kPa is measured to be 0.02 kg of water per kg of dry air. Then the partial pressure of water vapour in the air is:
- AP PGECET - 2025
- Thermodynamics
- If the vapour pressure at two temperatures of a solid phase in equilibrium with its liquid phase is known, then the latent heat of fusion can be calculated by the:
- AP PGECET - 2025
- Thermodynamics
- 1 mole of Argon gas is heated at constant pressure from 200 K to 600 K.
If \( C_p = 4\ \text{cal} \cdot \text{deg}^{-1} \cdot \text{mol}^{-1} \), then the change in entropy will be:
(Given \( \ln 3 = 1.09 \))
- AP PGECET - 2025
- Thermodynamics
- The minimum amount of work required to operate a refrigerator which removes 1000 Cal heat at $0^\circ$C and rejects at $50^\circ$C will be:
- AP PGECET - 2025
- Thermodynamics
- The entropy of single crystalline Silicon at absolute zero will be:
- AP PGECET - 2025
- Thermodynamics
View More Questions