An alkaline (NaOH) solution of a compound produces a yellow colored solution on addition of NaBO3. The compound is
- Mn(OH)2
- Pb(OH)2
- Cr(OH)3
- Fe(OH)3
The Correct Option is C
Solution and Explanation
The question pertains to identifying which compound produces a yellow-colored solution on reacting with an alkaline (NaOH) solution and NaBO3. Let's analyze the given options one by one:
- The compound Mn(OH)2: In alkaline solutions, manganous hydroxide does not participate in a reaction with NaBO3 to yield a yellow color. Therefore, this option is incorrect.
- The compound Pb(OH)2: Lead hydroxide in an alkaline environment also does not react with NaBO3 to produce a yellow-colored solution. Thus, this option is incorrect.
- The compound Cr(OH)3: Chromium hydroxide, in the presence of excess NaOH, forms chromate ions (CrO42-), which are yellow in color. The reaction with NaBO3 enhances the formation of these chromate ions, confirming the yellow coloration, making Cr(OH)3 the correct answer.
- The compound Fe(OH)3: Iron(III) hydroxide does not react with NaBO3 to produce a yellow solution in alkaline conditions. Therefore, this option is incorrect.
From this analysis, it is evident that the correct answer is Cr(OH)3. The chromate ions formed in alkaline conditions are responsible for the yellow coloration.
Top Questions on Qualitative Organic Analysis
- The major products X and Y in the following reactions
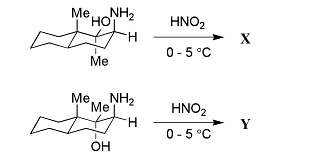
respectively, are- IIT JAM CY - 2024
- Organic Chemistry
- Qualitative Organic Analysis
- An organic compound having molecular formula $C_9H_{10}O_2$ exhibits the following spectral characteristics:
$'H NMR: \delta 9.72 (t, 1H), 7.1 (d, 2H), 6.7 (d, 2H), 3.8 (s, 3H), 3.6 (d, 2H)$
$IR: ~1720 cm^{-1}$
The most probable structure of the compound is- IIT JAM CY - 2022
- Organic Chemistry
- Qualitative Organic Analysis
- The reaction of 2,4-dinitrofluorobenzene with hydrazine produces a yellow orange solid X used for the identification of an organic functional group G. X and G, respectively, are
- IIT JAM CY - 2022
- Organic Chemistry
- Qualitative Organic Analysis
The compound(s) that shows(show) positive haloform test is(are)

- IIT JAM CY - 2018
- Organic Chemistry
- Qualitative Organic Analysis
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry