The compound(s) that shows(show) positive haloform test is(are)

The compound(s) that shows(show) positive haloform test is(are) 
Show Hint
- (A)
- (B)
- (C)
- (D)
The Correct Option is A, B
Solution and Explanation
The Requirement for a Positive Haloform Test
A compound gives a positive haloform test (reaction with $\text{X}_2$/$\text{OH}^-$ or $\text{I}_2$/$\text{NaOH}$, forming a haloform $\text{CHX}_3$) if it possesses one of the following structural units:
- Methyl Ketone Group: A methyl ketone group ($R-\text{CO}-\text{CH}_3$), where $R$ can be alkyl, aryl, or $\text{H}$ (except for formic acid, which doesn't react).
- Secondary Alcohol with a Methyl Group: A secondary alcohol with a methyl group adjacent to the $\text{-OH}$ bearing carbon ($\text{R-CH(OH)-CH}_3$). These are oxidized to methyl ketones under the reaction conditions before reacting further.
Analysis of the Options
We analyze each compound for the presence of the $\text{R-CO-CH}_3$ group:
(A) $p$-Methylacetophenone:
$$\text{Compound (A)} = \text{Ar}-\text{CO}-\text{CH}_3$$
This compound has a methyl ketone ($\text{CO}-\text{CH}_3$) group.
Therefore, Compound (A) shows a positive haloform test.
(B) Benzylidineacetone (4-Phenylbut-3-en-2-one):
$$\text{Compound (B)} = \text{Ar}-\text{CH}=\text{CH}-\text{CO}-\text{CH}_3$$
This compound also has a methyl ketone ($\text{CO}-\text{CH}_3$) group.
Therefore, Compound (B) shows a positive haloform test.
(C) Methyl $p$-Toluate:
$$\text{Compound (C)} = \text{Ar}-\text{CO}-\text{OCH}_3$$
This is an ester. It does not possess the $\text{CO}-\text{CH}_3$ group directly attached to the carbonyl carbon.
Therefore, Compound (C) does not show a positive haloform test.
(D) $p$-Methylstyrene (1-Propenylbenzene):
$$\text{Compound (D)} = \text{Ar}-\text{CH}=\text{CH}-\text{CH}_3$$
This is an alkene. It is neither a methyl ketone nor the required secondary alcohol structure.
Therefore, Compound (D) does not show a positive haloform test.
Conclusion
The compounds that show a positive haloform test are (A) and (B).
The correct answer is (A) and (B).
Top Questions on Qualitative Organic Analysis
- The major products X and Y in the following reactions
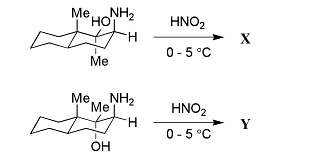
respectively, are- IIT JAM CY - 2024
- Organic Chemistry
- Qualitative Organic Analysis
- An alkaline (NaOH) solution of a compound produces a yellow colored solution on addition of NaBO3. The compound is
- IIT JAM CY - 2023
- Organic Chemistry
- Qualitative Organic Analysis
- An organic compound having molecular formula $C_9H_{10}O_2$ exhibits the following spectral characteristics:
$'H NMR: \delta 9.72 (t, 1H), 7.1 (d, 2H), 6.7 (d, 2H), 3.8 (s, 3H), 3.6 (d, 2H)$
$IR: ~1720 cm^{-1}$
The most probable structure of the compound is- IIT JAM CY - 2022
- Organic Chemistry
- Qualitative Organic Analysis
- The reaction of 2,4-dinitrofluorobenzene with hydrazine produces a yellow orange solid X used for the identification of an organic functional group G. X and G, respectively, are
- IIT JAM CY - 2022
- Organic Chemistry
- Qualitative Organic Analysis
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry