Question:
Which one of the following statement is true?
Which one of the following statement is true?
Updated On: Jun 14, 2022
- for monoatomic gases $ \gamma =1.40 $
- for diatomic gases $ {{C}_{p}}-{{C}_{V}}=2\text{ }cal $
- for polyatomic gases $ \gamma =1.67 $
- for all the gases $ {{C}_{p}}=4R $
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
: For diatomic gases, $ {{C}_{p}}-{{C}_{V}}=2\text{ }cal $
Was this answer helpful?
0
0
Top Questions on specific heat capacity
Match List-I with List-II.
List-I List-II (A) Heat capacity of body (I) \( J\,kg^{-1} \) (B) Specific heat capacity of body (II) \( J\,K^{-1} \) (C) Latent heat (III) \( J\,kg^{-1}K^{-1} \) (D) Thermal conductivity (IV) \( J\,m^{-1}K^{-1}s^{-1} \) - JEE Main - 2025
- Physics
- specific heat capacity
- Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
- KEAM - 2025
- Physics
- specific heat capacity
- If \( \frac{C_p}{C_v} \) is unity for a process, \( PV^{\gamma} = \text{constant} \), then the process is:
- KEAM - 2025
- Physics
- specific heat capacity
- Specific heat capacity of diatomic gas at constant volume:
- KEAM - 2025
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
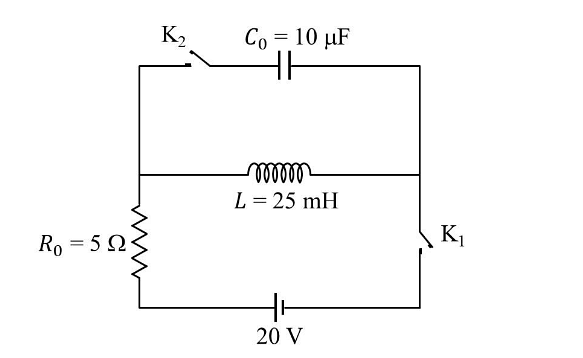
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
View More Questions
Questions Asked in AMUEEE exam
- The ratio of the half-life time $ (t_{1/2}) $ , to the three quarter life-time, $ (t_{3/4}) $ , for a reaction that is second order
- AMUEEE - 2018
- Order of Reaction
- $ 0.002\, M $ solution of a weak acid has an equivalent conductance $ (\wedge) 60\, ohm^{-1}\, cm^{2}\, eq^{-1} $ . What will be the $ pH $ ? (Given : $ \wedge = 400 \, ohm^{-1} \, cm^{2} \, eq^{-1} $ ]
- AMUEEE - 2018
- Electrochemistry
- The electrode potential, $ E^{\circ} $ , for the reduction of $ MnO^{-}_{4} $ to $ Mn^{2+} $ in acidic medium is $ +1.51\, V $ . Which of the following metal(s) will be oxidised? The reduction reactions and standard electrode potentials for $ Zn^{2+}, Ag^{+} $ , and $ Au^{+} $ are given as $ Zn^{2+} (aq) +2e \to Zn(s), E^{\circ} = -0.762\, V $ $ Ag+ (aq) +e {\rightleftharpoons} Ag (s), E^{\circ}=+0.80 \,V $ $ Au^{+} (aq) +e {\rightleftharpoons} Au(s), E^{\circ}+1.69\,V $
- AMUEEE - 2018
- Electrochemistry
- Three particles, two with masses $ m $ and one with mass $ M $ , might be arranged in any of the four configurations shown below. Rank the configurations according to the magnitude of the gravitational force on $ M $ , least to greatest

- AMUEEE - 2018
- Gravitation
- The $ EAN $ value $ y \left[Ti\left(\sigma-C_{6}H_{5}\right)_{2}\left(\pi-C_{5}H_{5}\right)_{2}\right]^{0} $ is
- AMUEEE - 2018
- coordination compounds
View More Questions
Concepts Used:
Specific Heat Capacity
Specific heat of a solid or liquid is the amount of heat that raises the temperature of a unit mass of the solid through 1°C.
Molar Specific Heat:
The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1°C.
Specific Heat at Constant Pressure or Volume:
The volume of solid remains constant when heated through a small range of temperature. This is known as specific heat at a constant volume. It is denoted as CV.
The pressure of solid remains constant when heated through a small range of temperature. This is known as specific heat at constant pressure which can be denoted as CP.