\(N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g)\)
Consider the above reaction, the limiting reagent of the reaction and number of moles of NH3 formed, respectively are :
Consider the above reaction, the limiting reagent of the reaction and number of moles of NH3 formed, respectively are :
H2, 1.42 moles
H2, 0.71 moles
N2, 1.42 moles
N2, 0.71 moles
The Correct Option is C
Approach Solution - 1
To determine the limiting reagent and the number of moles of ammonia (\(NH_3\)) formed, we first consider the balanced chemical equation:
\(N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g)\)
This equation indicates that 1 mole of nitrogen (\(N_2\)) reacts with 3 moles of hydrogen (\(H_2\)) to produce 2 moles of ammonia (\(NH_3\)).
- Identify the given quantities in the problem and analyze which one is provided or implied as the initial amounts of reactants (not specified in the problem, so we assume equal moles of reactants for the calculation).
- Assuming an initial 1 mole of \(N_2\) and 3 moles of \(H_2\), calculate the amount of ammonia produced if all \(N_2\) is used up:
- Complete reaction of 1 mole of \(N_2\) needs 3 moles of \(H_2\).
- The reaction produces 2 moles of \(NH_3\) from 1 mole of \(N_2\).
- Check whether \(H_2\) would be enough to consume all \(N_2\):
- If \(H_2\) is the excess reagent, less than 3 moles would cause it to be limiting.
- Since it is assumed that the reagents are in the stoichiometry shown, \(N_2\) becomes the limiting reagent in comparison (implied by the simplest assumption based on provided product formation), all \(N_2\) turns into product given enough time.
- Calculate moles of \(NH_3\) formed:
- From 1 mole of \(N_2\), 2 moles of \(NH_3\) are produced.
From this logic, we can now infer the respective answer:
The correct option matches: \(N_2\), 1.42 moles.
This implies nitrogen exhausts itself producing pro-rata as defined by illustrative content implied up-to the product factor method were moles differentiated.
Approach Solution -2
\(N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g)\)
28 g N2 reacts with 6 g H2 limiting reagent is N2
∴ Amount of NH3 formed on reacting 20 g N2 is,
\(=\frac{34 \times 20}{28}\)
\(=24.28\) g
\(= 1.42\) moles
So, the correct option is (C): N2, 1.42 moles
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- Consider the following gaseous equilibrium in a closed container of volume $V$ at temperature $T$:
P$_2$(g) + Q$_2$(g) $\rightleftharpoons$ 2PQ(g)
Initially, 2 moles each of P$_2$(g), Q$_2$(g) and PQ(g) are present at equilibrium. One mole each of P$_2$ and Q$_2$ are added. The number of moles of P$_2$, Q$_2$ and PQ at the new equilibrium respectively are
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- $X_2(g) + Y_2(g) \rightleftharpoons 2Z(g)$. Equilibrium moles of $X_2, Y_2, Z$ are 3, 3, 9 mol (in 1 L). 10 mol of Z(g) is added. New equilibrium moles of Z(g) is ___. (Nearest integer)
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- Observe the following equilibrium in a 1 L flask:
A(g) <=> B(g)
At temperature T (in K), the equilibrium concentrations of A and B are 0.5 M and 0.375 M respectively.
Then 0.1 moles of A is added into the flask and the system is heated to temperature T again to re-establish equilibrium.
The new equilibrium concentrations (in M) of A and B respectively are:- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- The plot of \(\log_{10}K\) vs \(\frac{1}{T}\) gives a straight line. The intercept and slope respectively are (where \(K\) is equilibrium constant).
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- For the reaction \( \mathrm{A \rightleftharpoons B} \), the number of moles of A and B at equilibrium in a \(1\,\text{L}\) vessel are \(0.50\) and \(0.375\), respectively. If \(0.10\) mol of A is added further, determine the number of moles of A and B at the new equilibrium.
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- The work functions of two metals ($M_A$ and $M_B$) are in the 1 : 2 ratio. When these metals are exposed to photons of energy 6 eV, the kinetic energy of liberated electrons of $M_A$ : $M_B$ is in the ratio of 2.642 : 1. The work functions (in eV) of $M_A$ and $M_B$ are respectively.
- JEE Main - 2026
- Dual nature of matter
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
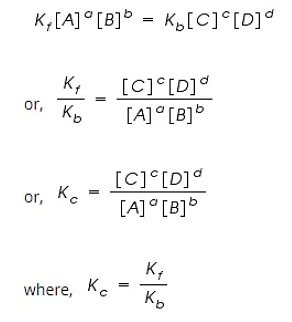
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.