Question:
Let . If , then is:
Let . If , then is:
Updated On: Jun 23, 2025
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
Explanation:
Given:We have to find the value of if Consider:After comparing, we get....(i)....(ii)Adding equation (i) and (ii)Hence, the correct option is (A).
Given:We have to find the value of if Consider:After comparing, we get....(i)....(ii)Adding equation (i) and (ii)Hence, the correct option is (A).
Was this answer helpful?
4
1
Top Questions on Transpose of a Matrix
- The variance of the following probability distribution is:
\[ \begin{array}{|c|c|} \hline x & P(X) \\ \hline 0 & \frac{9}{16} \\ 1 & \frac{3}{8} \\ 2 & \frac{1}{16} \\ \hline \end{array} \]- MHT CET - 2024
- Mathematics
- Transpose of a Matrix
- The negative of \( (p \land (\sim q)) \lor (\sim p) \) is equivalent to:
- MHT CET - 2024
- Mathematics
- Transpose of a Matrix
- The converse of \( ((\sim p) \land q) \Rightarrow r \) is:
- MHT CET - 2024
- Mathematics
- Transpose of a Matrix
- If \( A = \begin{bmatrix} 0 & 1 & 2 \\ 1 & 2 & 3 \\ 3 & 1 & 1 \end{bmatrix} \), then \( A^{-1} \) is:
- MHT CET - 2024
- Mathematics
- Transpose of a Matrix
- If \[ B = \begin{bmatrix} 3 & \alpha & -1 \\ 1 & 3 & 1 \\ -1 & 1 & 3 \end{bmatrix} \] is the adjoint of a 3x3 matrix \( A \) and \( |A| = 4 \), then \( \alpha \) is equal to:
- MHT CET - 2024
- Mathematics
- Transpose of a Matrix
View More Questions
Questions Asked in JEE Main exam
- Let $\vec{a}=2\hat{i}-\hat{j}-\hat{k}$, $\vec{b}=\hat{i}+3\hat{j}-\hat{k}$ and $\vec{c}=2\hat{i}+\hat{j}+3\hat{k}$. Let $\vec{v}$ be the vector in the plane of $\vec{a}$ and $\vec{b}$, such that the length of its projection on the vector $\vec{c}$ is $\dfrac{1}{\sqrt{14}}$. Then $|\vec{v}|$ is equal to
- JEE Main - 2026
- Vector Algebra
- A 20 m long uniform copper wire held horizontally is allowed to fall under the gravity (g = 10 m/s²) through a uniform horizontal magnetic field of 0.5 Gauss perpendicular to the length of the wire. The induced EMF across the wire when it travels a vertical distance of 200 m is_______ mV.}
- JEE Main - 2026
- Thermodynamics
- If the end points of chord of parabola \(y^2 = 12x\) are \((x_1, y_1)\) and \((x_2, y_2)\) and it subtend \(90^\circ\) at the vertex of parabola then \((x_1x_2 - y_1y_2)\) equals :
- JEE Main - 2026
- Probability
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
View More Questions
Concepts Used:
Transpose of a Matrix
The matrix acquired by interchanging the rows and columns of the parent matrix is called the Transpose matrix. The transpose matrix is also defined as - “A Matrix which is formed by transposing all the rows of a given matrix into columns and vice-versa.”
The transpose matrix of A is represented by A’. It can be better understood by the given example:

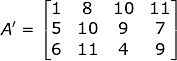
Now, in Matrix A, the number of rows was 4 and the number of columns was 3 but, on taking the transpose of A we acquired A’ having 3 rows and 4 columns. Consequently, the vertical Matrix gets converted into Horizontal Matrix.
Hence, we can say if the matrix before transposing was a vertical matrix, it will be transposed to a horizontal matrix and vice-versa.
Read More: Transpose of a Matrix