In Carius method of estimation of halogen, 0.45 g of an organic compound gave 0.36 g of AgBr. Find out the percentage of bromine in the compound.
(Molar masses: AgBr = 188 g mol–1; Br = 80 g mol–1)
(Molar masses: AgBr = 188 g mol–1; Br = 80 g mol–1)
- 34.04%
- 40.04%
- 36.03%
- 38.04%
The Correct Option is A
Solution and Explanation
The correct answer is (A):
188g of AgBr = 80g of Br
0.36g of AgBr = \(\frac{80}{188}\)×0.36
Percentage of Br in given organic compound
= 80×\(\frac{0.36}{188}\)×0.45×100
= 34.04%
Top Questions on introduction to organic chemistry
- Which of the following represents the correct IUPAC name for \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \)?
- MHT CET - 2025
- Chemistry
- introduction to organic chemistry
- Which of the following represents the correct IUPAC name for \( \text{CH}_3\text{CH}_2\text{OH} \)?
- MHT CET - 2025
- Chemistry
- introduction to organic chemistry
- What is the IUPAC name of CH$_3$CH(OH)CH$_3$?
- BITSAT - 2025
- Chemistry
- introduction to organic chemistry
- What is the concentration of \( \text{H}^+ \) ions if the pH is 2.7?
- MHT CET - 2024
- Chemistry
- introduction to organic chemistry
- Calculate the pH of the solution using the Henderson-Hasselbalch equation.
- MHT CET - 2024
- Chemistry
- introduction to organic chemistry
Questions Asked in JEE Main exam
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
Concepts Used:
Types of Differential Equations
There are various types of Differential Equation, such as:
Ordinary Differential Equations:
Ordinary Differential Equations is an equation that indicates the relation of having one independent variable x, and one dependent variable y, along with some of its other derivatives.
\(F(\frac{dy}{dt},y,t) = 0\)
Partial Differential Equations:
A partial differential equation is a type, in which the equation carries many unknown variables with their partial derivatives.
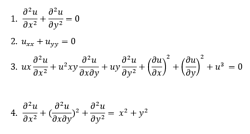
Linear Differential Equations:
It is the linear polynomial equation in which derivatives of different variables exist. Linear Partial Differential Equation derivatives are partial and function is dependent on the variable.
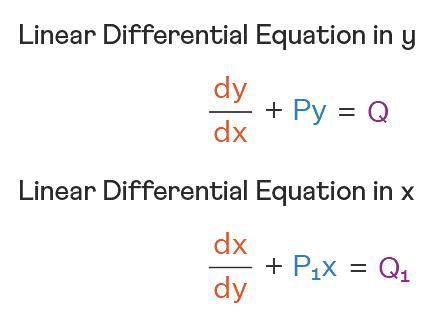
Homogeneous Differential Equations:
When the degree of f(x,y) and g(x,y) is the same, it is known to be a homogeneous differential equation.
\(\frac{dy}{dx} = \frac{a_1x + b_1y + c_1}{a_2x + b_2y + c_2}\)
Read More: Differential Equations