Amongst FeCl3.3H2O, K3[Fe(CN)6)] and [Co(NH3)6]Cl3, the spin-only magnetic moment value of the inner-orbital complex that absorbs light at shortest wavelength is_______B.M. [nearest integer]
Correct Answer: 2
Solution and Explanation
[Fe(H2O)3Cl3] → Outer-orbital complex
K3[Fe(CN)6] → Inner-orbital complex
[Co(NH3)6]Cl3 → Inner-orbital complex
Since CN– is a strong field ligand than NH3. Hence K3[Fe(CN)6] is the inner-orbital complex that absorbs light at shortest wavelength.
Fe(III) → valence shell configuration 3d5
Since CN– will do pairing, so unpaired electron = 1
\(μ=\sqrt{1(1+2)}=\sqrt3BM=2BM\)
Top Questions on Chemical Reactions of Alcohols Phenols and Ethers
- Phenol can be distinguished from propanol by using the reagent
- KCET - 2025
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
Calculate the potential for half-cell containing 0.01 M K\(_2\)Cr\(_2\)O\(_7\)(aq), 0.01 M Cr\(^{3+}\)(aq), and 1.0 x 10\(^{-4}\) M H\(^+\)(aq).
- CBSE CLASS XII - 2025
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Number of isomeric products formed by monochlorination Of \(2-methyl \) \(butane\) in presence of sunlight is
- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Find out the final product C

- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Moles of \(CH_4\) required for formation of \(22\) \(g\) of \(CO_2\) is \(m \times 10^{-2}\) The value of \(m\) is:
- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
Questions Asked in JEE Main exam
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Concepts Used:
Types of Differential Equations
There are various types of Differential Equation, such as:
Ordinary Differential Equations:
Ordinary Differential Equations is an equation that indicates the relation of having one independent variable x, and one dependent variable y, along with some of its other derivatives.
\(F(\frac{dy}{dt},y,t) = 0\)
Partial Differential Equations:
A partial differential equation is a type, in which the equation carries many unknown variables with their partial derivatives.
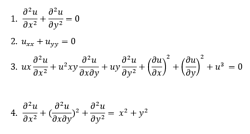
Linear Differential Equations:
It is the linear polynomial equation in which derivatives of different variables exist. Linear Partial Differential Equation derivatives are partial and function is dependent on the variable.
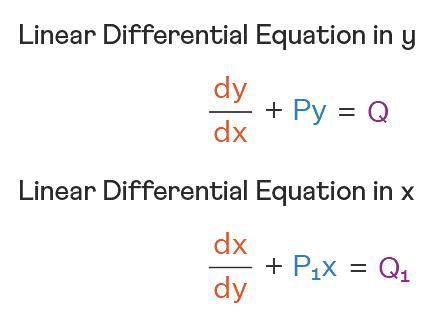
Homogeneous Differential Equations:
When the degree of f(x,y) and g(x,y) is the same, it is known to be a homogeneous differential equation.
\(\frac{dy}{dx} = \frac{a_1x + b_1y + c_1}{a_2x + b_2y + c_2}\)
Read More: Differential Equations