Which of the following free radicals helps in depletion of the ozone layer?
- NO
- Cl
- OH
- CH3
The Correct Option is B
Approach Solution - 1
The correct option is (B): Cl
The depletion of the ozone layer is mainly caused by the presence of free radicals containing chlorine and/or bromine atoms, which are collectively referred to as halogen radicals.
The two most important halogen radicals responsible for the depletion of the ozone layer are:
- Chlorine radical (Cl•)
- Bromine radical (Br•)
These free radicals are formed from the breakdown of chlorofluorocarbons (CFCs) and other halogenated compounds, which were widely used as refrigerants, propellants, and solvents before their harmful effects on the ozone layer were discovered.
When CFCs and other halogenated compounds are released into the atmosphere, they are carried up into the stratosphere by natural air currents. In the stratosphere, the compounds are broken down by the intense ultraviolet radiation from the sun, releasing chlorine and/or bromine atoms as free radicals.
Once released, these halogen radicals can react with ozone (O3) molecules, breaking them down into oxygen (O2) molecules and leaving behind more free radicals to continue the cycle of ozone destruction. This process is known as ozone depletion and leads to the thinning of the ozone layer.
Therefore, the free radicals that help in the depletion of the ozone layer are the halogen radicals, particularly the chlorine and bromine radicals.
Approach Solution -2

Top Questions on Environmental Chemistry
- Minamata disease is caused by .......... poisoning.
- CUET (PG) - 2025
- Chemistry
- Environmental Chemistry
- Which of the following is dominantly responsible for buffering capacity of natural water?
- CUET (PG) - 2025
- Atmospheric Science
- Environmental Chemistry
- Which one of the following are the commonly used oxidants in COD (Chemical Oxygen Demand) assays?
- CUET (UG) - 2025
- Environmental Science
- Environmental Chemistry
- The octet rule is not valid for which one of the following molecules?
- LPUNEST - 2025
- Chemistry
- Environmental Chemistry
- Polyhalogen compounds have wide application in industries and agriculture. DDT is also a very important polyhalogen compound. It is a:
- CBSE CLASS XII - 2025
- Chemistry
- Environmental Chemistry
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- For two identical cells each having emf \(E\) and internal resistance \(r\), the current through an external resistor of \(6\,\Omega\) is the same when the cells are connected in series as well as in parallel. The value of the internal resistance \(r\) is ________ \(\Omega\).
- JEE Main - 2026
- Current electricity
- For three unit vectors \( \vec a, \vec b, \vec c \) satisfying \[ |\vec a-\vec b|^2 + |\vec b-\vec c|^2 + |\vec c-\vec a|^2 = 9 \] and \[ |2\vec a + k\vec b + k\vec c| = 3, \] the positive value of \( k \) is:
- JEE Main - 2026
- Vector Algebra
In the given figure, the blocks $A$, $B$ and $C$ weigh $4\,\text{kg}$, $6\,\text{kg}$ and $8\,\text{kg}$ respectively. The coefficient of sliding friction between any two surfaces is $0.5$. The force $\vec{F}$ required to slide the block $C$ with constant speed is ___ N.
(Given: $g = 10\,\text{m s}^{-2}$)
- JEE Main - 2026
- Rotational Mechanics
- The system of linear equations
$x + y + z = 6$
$2x + 5y + az = 36$
$x + 2y + 3z = b$
has- JEE Main - 2026
- Matrices and Determinants
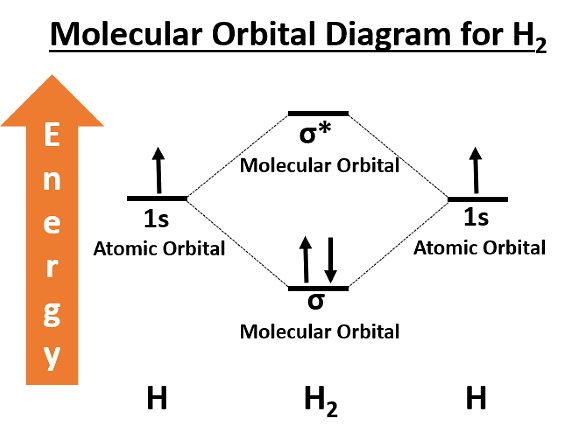
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.