Two identical bodies are made of a material for which the heat capacity increases with temperature. One of these is at $100^{\circ} C$ , while the other one is at $0^{\circ} C$. If the two bodies are brought into contact, then, assuming no heat loss, the final common temperature is -
- $50^{\circ} C$
- more than $50^{\circ} C$
- less than $50^{\circ} C$ but greater than $0^{\circ} C$
- $0^{\circ} C$
The Correct Option is B
Solution and Explanation
Heat Capacity and Temperature Relationship
As the temperature increases, the heat capacity also increases. Hence, the heat capacity of the second substance is greater than the first. Let the heat capacities be \( c_1 \) and \( c_2 \) for the first and second substances, respectively, and let the common temperature be \( T \). The equation for heat transfer is:
\(mc_1 (T - 0) = mc_2 (100 - T)\)
We can simplify this equation to:
\(\frac{T}{100 - T} = \frac{c_2}{c_1} > 1\)
This implies:
\(c_2 > c_1\)
Since \( \frac{T}{100 - T} > 1 \), it follows that:
\(T > 50\)
Conclusion:
The temperature \( T \) must be greater than 50 for the heat capacity of the second substance to be greater than the first.
Top Questions on specific heat capacity
Match List-I with List-II.
List-I List-II (A) Heat capacity of body (I) \( J\,kg^{-1} \) (B) Specific heat capacity of body (II) \( J\,K^{-1} \) (C) Latent heat (III) \( J\,kg^{-1}K^{-1} \) (D) Thermal conductivity (IV) \( J\,m^{-1}K^{-1}s^{-1} \) - JEE Main - 2025
- Physics
- specific heat capacity
- Product of uncertainty in position (\(\Delta x\)) and uncertainty in velocity (\(\Delta v\)) has unit:
- KEAM - 2025
- Physics
- specific heat capacity
- If \( \frac{C_p}{C_v} \) is unity for a process, \( PV^{\gamma} = \text{constant} \), then the process is:
- KEAM - 2025
- Physics
- specific heat capacity
- Specific heat capacity of diatomic gas at constant volume:
- KEAM - 2025
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
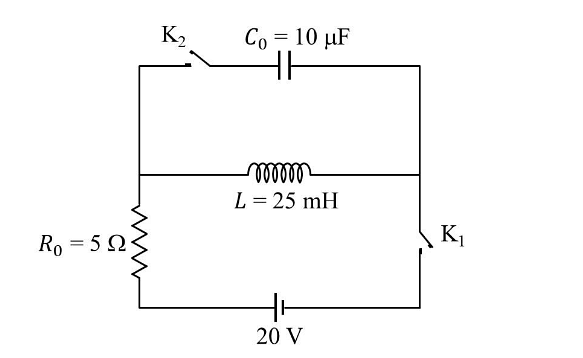
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
Concepts Used:
Specific Heat Capacity
Specific heat of a solid or liquid is the amount of heat that raises the temperature of a unit mass of the solid through 1°C.
Molar Specific Heat:
The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1°C.
Specific Heat at Constant Pressure or Volume:
The volume of solid remains constant when heated through a small range of temperature. This is known as specific heat at a constant volume. It is denoted as CV.
The pressure of solid remains constant when heated through a small range of temperature. This is known as specific heat at constant pressure which can be denoted as CP.