The volume of water (in mL) required to be added to a 100 mL solution (aq. 0.1 M) of a weak acid (HA) at 25 °C to double its degree of dissociation is
[Given: Ka of HA at 25 °C=1.8 x10−5 ]
[Given: Ka of HA at 25 °C=1.8 x10−5 ]
- 100
- 200
- 300
- 400
The Correct Option is C
Solution and Explanation
To solve this problem, we need to determine how much additional water should be added to a weak acid solution to double its degree of dissociation.
Given:
- Initial concentration of HA, \([HA]_0 = 0.1 \, \text{M}\)
- Degree of dissociation initially, \(\alpha_1\)
- Dissociation constant, \(K_a = 1.8 \times 10^{-5}\)
The degree of dissociation, \(\alpha\), for a weak acid is given by:
\(\alpha = \sqrt{\frac{K_a}{[HA]_0}}\)
Initial degree of dissociation is:
\(\alpha_1 = \sqrt{\frac{1.8 \times 10^{-5}}{0.1}}\)
\(\alpha_1 = \sqrt{1.8 \times 10^{-4}} = 0.0134\)
We want to double the degree of dissociation:
\(\alpha_2 = 2\alpha_1 = 2 \times 0.0134 = 0.0268\)
The final concentration of the acid after dilution should make the new degree of dissociation equal to \(\alpha_2\):
\(\alpha_2 = \sqrt{\frac{K_a}{[HA]_2}}\)
\(0.0268 = \sqrt{\frac{1.8 \times 10^{-5}}{[HA]_2}}\)
Squaring both sides gives:
\(0.00071824 = \frac{1.8 \times 10^{-5}}{[HA]_2}\)
\([HA]_2 = \frac{1.8 \times 10^{-5}}{0.00071824} = 0.0251 \, \text{M}\)
The concentration changes with dilution. Using the dilution formula:
\(C_1 V_1 = C_2 V_2\)
\(0.1 \times 100 = 0.0251 \times V_2\)
\(V_2 = \frac{10}{0.0251} = 398.41 \, \text{mL}\)
The volume of water to be added is:
\(V_2 - 100 \, \text{mL} = 398.41 - 100 = 298.41 \, \text{mL}\)
Since we need to choose the closest option: 300 mL is the correct volume to add.
Therefore, the correct answer is 300 mL.
Top Questions on Chemical equilibria
- The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- A 1.0 L solution is prepared by dissolving 2.0 g of benzoic acid and 4.0 g of sodium benzoate in water. The pH of the resulting solution is _______. (rounded off to one decimal place)
Given: Molar mass of benzoic acid is 122 g mol−1
Molar mass of sodium benzoate is 144 g mol−1
p𝐾a of benzoic acid is 4.2- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- 0.1 M aqueous solution of a weak monobasic acid has pH 2.0. The pKa of the monobasic acid is _______. (rounded off to one decimal place)
- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- Consider the exothermic chemical reaction O2(𝑔)+2H2(𝑔) ⇌ 2H2O(𝑔) at equilibrium in a closed container. The correct statement(s) is/are
- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- The isoelectric point of glutamic acid is ______.
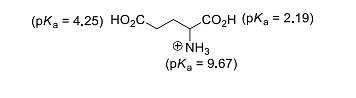
(round off to two decimal places)- IIT JAM CY - 2023
- Physical Chemistry
- Chemical equilibria
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry