The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]
[Given: Both compounds dissociate completely in water]
- 3:1
- 1:4
1:1
- 3:2
The Correct Option is A
Solution and Explanation
Osmotic pressure (π) is directly proportional to the molar concentration of the solute and the number of ions produced when the solute dissociates.
- BaCl2 dissociates into 3 ions (Ba2+, 2Cl−) per formula unit, so the effective concentration of ions is 0.01 × 3 = 0.03 M.
- NaCl dissociates into 2 ions (Na+, Cl−) per formula unit, so the effective concentration of ions is 0.005 × 2 = 0.01 M.
Therefore, the ratio of osmotic pressures is:
\( \frac{\text{osmotic pressure of BaCl}_2}{\text{osmotic pressure of NaCl}} = \frac{0.03}{0.01} = 3\)
Thus, the correct answer is (A). 3:1
Top Questions on Chemical equilibria
- A 1.0 L solution is prepared by dissolving 2.0 g of benzoic acid and 4.0 g of sodium benzoate in water. The pH of the resulting solution is _______. (rounded off to one decimal place)
Given: Molar mass of benzoic acid is 122 g mol−1
Molar mass of sodium benzoate is 144 g mol−1
p𝐾a of benzoic acid is 4.2- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- 0.1 M aqueous solution of a weak monobasic acid has pH 2.0. The pKa of the monobasic acid is _______. (rounded off to one decimal place)
- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- Consider the exothermic chemical reaction O2(𝑔)+2H2(𝑔) ⇌ 2H2O(𝑔) at equilibrium in a closed container. The correct statement(s) is/are
- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- The isoelectric point of glutamic acid is ______.
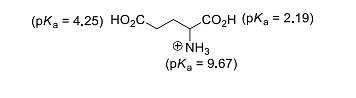
(round off to two decimal places)- IIT JAM CY - 2023
- Physical Chemistry
- Chemical equilibria
- The volume of water (in mL) required to be added to a 100 mL solution (aq. 0.1 M) of a weak acid (HA) at 25 °C to double its degree of dissociation is
[Given: Ka of HA at 25 °C=1.8 x10−5 ]- IIT JAM CY - 2023
- Physical Chemistry
- Chemical equilibria
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry