Question:
Consider the exothermic chemical reaction O2(𝑔)+2H2(𝑔) ⇌ 2H2O(𝑔) at equilibrium in a closed container. The correct statement(s) is/are
Consider the exothermic chemical reaction O2(𝑔)+2H2(𝑔) ⇌ 2H2O(𝑔) at equilibrium in a closed container. The correct statement(s) is/are
Updated On: Jan 11, 2025
- At equilibrium, introduction of catalyst increases product formation
- Equilibrium constant decreases with increase in temperature.
- The equilibrium constant 𝐾𝑃 increases with pressure.
- Decrease in volume of reaction vessel increases product formation.
Hide Solution
Verified By Collegedunia
The Correct Option is B, D
Solution and Explanation
- (A) A catalyst only affects the rate of reaction, not the equilibrium position. Therefore, the introduction of a catalyst does not increase product formation at equilibrium.
- (B) For an exothermic reaction, increasing the temperature shifts the equilibrium toward the reactants (Le Chatelier’s Principle). Thus, the equilibrium constant decreases with an increase in temperature.
- (C) The equilibrium constant Kp is not directly affected by pressure for reactions involving gases unless there is a change in the number of moles of gas. Since this reaction has no change in the number of moles of gas, Kp remains unaffected by pressure.
- (D) According to Le Chatelier’s Principle, reducing the volume of a reaction vessel increases the pressure and shifts the equilibrium toward the side with fewer moles of gas. In this case, the product side (2 moles of H2O) has fewer moles of gas than the reactant side (3 moles of gas), so decreasing the volume increases product formation.
Thus, the correct answers are (B) and (D)
Was this answer helpful?
0
0
Top Questions on Chemical equilibria
- The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- A 1.0 L solution is prepared by dissolving 2.0 g of benzoic acid and 4.0 g of sodium benzoate in water. The pH of the resulting solution is _______. (rounded off to one decimal place)
Given: Molar mass of benzoic acid is 122 g mol−1
Molar mass of sodium benzoate is 144 g mol−1
p𝐾a of benzoic acid is 4.2- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- 0.1 M aqueous solution of a weak monobasic acid has pH 2.0. The pKa of the monobasic acid is _______. (rounded off to one decimal place)
- IIT JAM CY - 2024
- Physical Chemistry
- Chemical equilibria
- The isoelectric point of glutamic acid is ______.
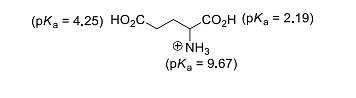
(round off to two decimal places)- IIT JAM CY - 2023
- Physical Chemistry
- Chemical equilibria
- The volume of water (in mL) required to be added to a 100 mL solution (aq. 0.1 M) of a weak acid (HA) at 25 °C to double its degree of dissociation is
[Given: Ka of HA at 25 °C=1.8 x10−5 ]- IIT JAM CY - 2023
- Physical Chemistry
- Chemical equilibria
View More Questions
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry
View More Questions