Question:
The variation of stopping potential (V0) as a function of the frequency (v) of the incident light for a metal is shown in figure. The work function of the surface is
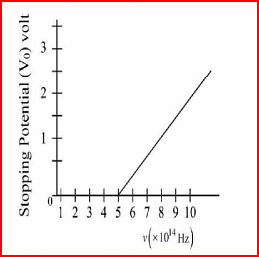
The variation of stopping potential (V0) as a function of the frequency (v) of the incident light for a metal is shown in figure. The work function of the surface is


Updated On: Feb 2, 2026
- 1.36 eV
- 2.98 eV
- 2.07 eV
- 18.6 eV
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
Step 1: Understanding the photoelectric equation.
In the photoelectric effect, the stopping potential (\( V_0 \)) is related to the frequency (\( v \)) of the incident light by the equation: \[ eV_0 = h(v - v_{\text{th}}) \] Where:
\( V_0 \) is the stopping potential (in volts).
\( v \) is the frequency of the incident light (in Hz).
\( v_{\text{th}} \) is the threshold frequency (below which no photoelectric emission occurs).
\( e \) is the charge of the electron (\( 1.6 \times 10^{-19} \, \text{C} \)).
\( h \) is Planck's constant (\( 6.6 \times 10^{-34} \, \text{J} \cdot \text{s} \)).
Step 2: Identifying the threshold frequency.
From the graph, we can observe that the stopping potential becomes non-zero at a frequency of approximately \( 5 \times 10^{14} \, \text{Hz} \). This is the threshold frequency \( v_{\text{th}} \).
Step 3: Calculating the work function.
At the threshold frequency, the stopping potential is zero. We use the equation: \[ \phi = h v_{\text{th}} \] Substituting the values: \[ \phi = (6.6 \times 10^{-34}) \times (5 \times 10^{14}) = 33 \times 10^{-20} \, \text{J} \] \[ \phi = 3.3 \times 10^{-19} \, \text{J} \] To convert this to eV, divide by the charge of the electron: \[ \phi = \frac{3.3 \times 10^{-19}}{1.6 \times 10^{-19}} \, \text{eV} = 2.07 \, \text{eV} \] Thus, the work function is \( \phi = 2.07 \, \text{eV} \).
In the photoelectric effect, the stopping potential (\( V_0 \)) is related to the frequency (\( v \)) of the incident light by the equation: \[ eV_0 = h(v - v_{\text{th}}) \] Where:
\( V_0 \) is the stopping potential (in volts).
\( v \) is the frequency of the incident light (in Hz).
\( v_{\text{th}} \) is the threshold frequency (below which no photoelectric emission occurs).
\( e \) is the charge of the electron (\( 1.6 \times 10^{-19} \, \text{C} \)).
\( h \) is Planck's constant (\( 6.6 \times 10^{-34} \, \text{J} \cdot \text{s} \)).
Step 2: Identifying the threshold frequency.
From the graph, we can observe that the stopping potential becomes non-zero at a frequency of approximately \( 5 \times 10^{14} \, \text{Hz} \). This is the threshold frequency \( v_{\text{th}} \).
Step 3: Calculating the work function.
At the threshold frequency, the stopping potential is zero. We use the equation: \[ \phi = h v_{\text{th}} \] Substituting the values: \[ \phi = (6.6 \times 10^{-34}) \times (5 \times 10^{14}) = 33 \times 10^{-20} \, \text{J} \] \[ \phi = 3.3 \times 10^{-19} \, \text{J} \] To convert this to eV, divide by the charge of the electron: \[ \phi = \frac{3.3 \times 10^{-19}}{1.6 \times 10^{-19}} \, \text{eV} = 2.07 \, \text{eV} \] Thus, the work function is \( \phi = 2.07 \, \text{eV} \).
Was this answer helpful?
0
1
Top Questions on work, energy and power
- Given below are two statements:
Statement I: An object moves from position \( \vec{r}_1 \) to position \( \vec{r}_2 \) under a conservative force field \( \vec{F} \). The work done by the force is \[ W = -\int_{\vec{r}_1}^{\vec{r}_2} \vec{F} \cdot d\vec{r}. \]
Statement II: Any object moving from one location to another location can follow infinite number of paths. Therefore, the amount of work done by the object changes with the path it follows for a conservative force.
In the light of the above statements, choose the correct answer from the options given below:- JEE Main - 2026
- Physics
- work, energy and power
- Two masses \(m\) and \(2m\) are connected by a light string going over a pulley (disc) of mass \(30m\) with radius \(r=0.1\,\text{m}\). The pulley is mounted in a vertical plane and is free to rotate about its axis. The \(2m\) mass is released from rest and its speed when it has descended through a height of \(3.6\,\text{m}\) is ____________ m/s. (Assume string does not slip and \(g=10\,\text{m s}^{-2}\)).
- JEE Main - 2026
- Physics
- work, energy and power
- A large drum having radius $R$ is spinning around its axis with angular velocity $\omega$, as shown in figure. The minimum value of $\omega$ so that a body of mass $M$ remains stuck to the inner wall of the drum, taking the coefficient of friction between the drum surface and mass $M$ as $\mu$, is :

- JEE Main - 2026
- Physics
- work, energy and power
Potential energy (V) versus distance (x) is given by the graph. Rank various regions as per the magnitudes of the force (F) acting on a particle from high to low.

- JEE Main - 2026
- Physics
- work, energy and power
- A body of mass 4 kg is placed on a plane at a point P having coordinate $(3,4)$ m. Under the action of force $\vec{F} = (2\hat{i} + 3\hat{j})$ N, it moves to a new point Q having coordinates $(6,10)$ m in 4 sec. The average power and instantaneous power at the end of 4 sec are in the ratio of:
- JEE Main - 2026
- Physics
- work, energy and power
View More Questions
Questions Asked in JEE Main exam
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
View More Questions