The number of bent-shaped molecule(s) from the following is_______?
SO2, O3, I3-, N3-
SO2, O3, I3-, N3-
Solution and Explanation
1. SO2 (Sulfur Dioxide):
SO2 has a bent molecular geometry due to the lone pair of electrons on the sulfur atom, which causes electron pair repulsion and distorts the shape.
2. O3 (Ozone):
O3 has a central oxygen atom surrounded by one lone pair and three bonding electrons. This results in a bent molecular geometry, as the lone pair causes distortion in the structure.
3. I3- (Triiodide Ion):
I3- has a linear geometry. The central iodine atom has three lone pairs, and the bonding electrons are delocalized, maintaining a linear structure.
4. N3- (Azide Ion):
N3- is a linear molecule, as it consists of a chain of three nitrogen atoms connected by alternating double and single bonds, with delocalization of electrons across the structure.
Conclusion:
- Bent: SO2, O3
- Linear: I3-, N3-
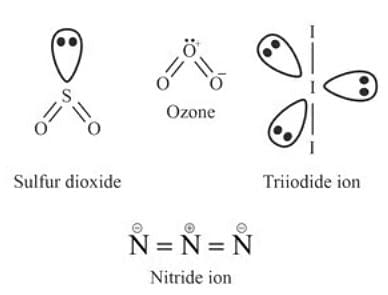
So, Two molecules/ions, SO2 and O3, have a bent shape. The other two, I3- and N3-, have a linear form.
Top Questions on coordination compounds
Given below are two statements regarding conformations of n-butane. Choose the correct option.

- JEE Main - 2026
- Chemistry
- coordination compounds
- The correct statement among the following is:
- JEE Main - 2026
- Chemistry
- coordination compounds
Consider a weak base \(B\) of \(pK_b = 5.699\). \(x\) mL of \(0.02\) M HCl and \(y\) mL of \(0.02\) M weak base \(B\) are mixed to make \(100\) mL of a buffer of pH \(=9\) at \(25^\circ\text{C}\). The values of \(x\) and \(y\) respectively are
- JEE Main - 2026
- Chemistry
- coordination compounds
- \(20.0\,\text{dm}^3\) of an ideal gas \(X\) at \(600\) K and \(0.5\) MPa undergoes isothermal reversible expansion until the pressure of the gas becomes \(0.2\) MPa. Which of the following option is correct? (Given: \(\log 2 = 0.3010\), \(\log 5 = 0.6989\))
- JEE Main - 2026
- Chemistry
- coordination compounds
- From the following : (A) $[\text{Co}(\text{NH}_3)_6]^{3+}$ : Inner orbital complex, $d^2sp^3$ hybridization (B) $[\text{MnCl}_4]^{2-}$ : Outer orbital complex, $sp^3d^2$ hybridization (C) $[\text{CoF}_6]^{3-}$ : Outer orbital complex, $d^2sp^3$ hybridization (D) $[\text{FeF}_6]^{3-}$ : Outer orbital complex, $sp^3d^2$ hybridization (E) $[\text{Ni}(\text{CN})_4]^{2-}$ : Inner orbital complex, $sp^3$ hybridization Choose the correct answer from the given options.
- JEE Main - 2026
- Chemistry
- coordination compounds
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- The work functions of two metals ($M_A$ and $M_B$) are in the 1 : 2 ratio. When these metals are exposed to photons of energy 6 eV, the kinetic energy of liberated electrons of $M_A$ : $M_B$ is in the ratio of 2.642 : 1. The work functions (in eV) of $M_A$ and $M_B$ are respectively.
- JEE Main - 2026
- Dual nature of matter
Concepts Used:
Coordination Compounds
A coordination compound holds a central metal atom or ion surrounded by various oppositely charged ions or neutral molecules. These molecules or ions are re-bonded to the metal atom or ion by a coordinate bond.
Coordination entity:
A coordination entity composes of a central metal atom or ion bonded to a fixed number of ions or molecules.
Ligands:
A molecule, ion, or group which is bonded to the metal atom or ion in a complex or coordination compound by a coordinate bond is commonly called a ligand. It may be either neutral, positively, or negatively charged.