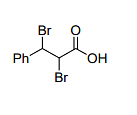
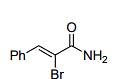
The major product formed in the following reaction
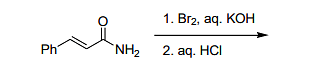
is
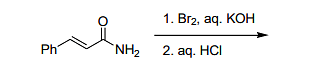
is
The Correct Option is B
Solution and Explanation
Step 1: Bromination of the amino group on the aromatic ring will lead to the formation of a bromo-phenyl group at the para position with respect to the amino group.
Step 2: The reaction with aqueous KOH will induce nucleophilic substitution, replacing the bromine with a hydrogen atom.
Therefore, the final product is Ph–CH2–H (option B).
Thus, the correct answer is B
The correct option is (B) : 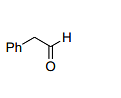
Top Questions on Reaction Mechanisms & Synthesis
- The reaction represented by \( A \rightarrow B \) follows first-order kinetics. At a given temperature, 20% of the reaction is completed in 223 s. The time taken to complete 50% of the reaction at the same temperature is _________ s (rounded off to the nearest integer).
- GATE MT - 2025
- Mineral Processing and Extractive Metallurgy
- Reaction Mechanisms & Synthesis
- Consider the following reactions and their standard Gibbs free energies (in J): \[ {Fe(s)} + \frac{1}{2} {O}_2(g) \rightleftharpoons {FeO(s)} \quad \Delta G^\circ = -264900 + 65T \] \[ 2 {H}_2(g) + {O}_2(g) \rightleftharpoons 2 {H}_2{O(g)} \quad \Delta G^\circ = -492900 + 109T \] Assuming Fe and FeO to be pure and no solubility of gases in the solids, the value of \( \frac{p_{H_2O}}{p_{H_2}} \) required to reduce solid FeO to solid Fe at 1000 K is _________ (rounded off to two decimal places). Given: Ideal gas constant \( R = 8.314 \, {J mol}^{-1} {K}^{-1} \).
- GATE MT - 2025
- Mineral Processing and Extractive Metallurgy
- Reaction Mechanisms & Synthesis
- Molten steel at 1900 K having dissolved hydrogen needs to be vacuum degassed. The equilibrium partial pressure of hydrogen to be maintained to achieve 1 ppm (mass basis) of dissolved hydrogen is ......... Torr (rounded off to two decimal places). Given: For the hydrogen dissolution reaction in molten steel \( \left( \frac{1}{2} {H}_2(g) = [{H}] \right) \), the equilibrium constant (expressed in terms of ppm of dissolved H) is: \[ \log_{10} K_{eq} = \frac{1900}{T} + 2.4 \] 1 atm = 760 Torr.
- GATE MT - 2025
- Mineral Processing and Extractive Metallurgy
- Reaction Mechanisms & Synthesis
- Consider the gas phase reaction: \[ CO + \frac{1}{2} O_2 \rightleftharpoons CO_2 \] At equilibrium for a particular temperature, the partial pressures of \( CO \), \( O_2 \), and \( CO_2 \) are found to be \( 10^{-6} \, {atm} \), \( 10^{-6} \, {atm} \), and \( 16 \, {atm} \), respectively. The equilibrium constant for the reaction is ......... \( \times 10^{10} \) (rounded off to one decimal place).
- GATE MT - 2025
- Mineral Processing and Extractive Metallurgy
- Reaction Mechanisms & Synthesis
- In the Claisen-Schmidt reaction to prepare dibenzalacetone from 5.3 g benzaldehyde, a total of 3.51 g of product was obtained. The percentage yield in this reaction was _____.
- JEE Main - 2025
- Chemistry
- Reaction Mechanisms & Synthesis
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry