Question:
The half-life period of a first order reaction is 1000 seconds. Its rate constant is:
The half-life period of a first order reaction is 1000 seconds. Its rate constant is:
Show Hint
For first-order reactions, the half-life is inversely proportional to the rate constant.
Updated On: Mar 7, 2025
- \(0.693 \, {sec}^{-1}\)
- \(6.93 \times 10^{-2} \, {sec}^{-1}\)
- \(6.93 \times 10^{-3} \, {sec}^{-1}\)
- \(6.93 \times 10^{-4} \, {sec}^{-1}\)
- \(6.93 \times 10^{-1} \, {sec}^{-1}\)
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
For a first-order reaction, the relationship between half-life (\(t_{1/2}\)) and rate constant (\(k\)) is given by:
\[
t_{1/2} = \frac{0.693}{k}
\]
Substituting the given half-life (\(1000 \, {sec}\)):
\[
1000 = \frac{0.693}{k}
\]
Solving for \(k\):
\[
k = \frac{0.693}{1000} = 6.93 \times 10^{-4} \, {sec}^{-1}
\]
Was this answer helpful?
0
0
Top Questions on kinetics equations
- Magnetic Moment of \( \text{Mn}^{2+} \) is:
- MHT CET - 2024
- Chemistry
- kinetics equations
Find the time required to complete a reaction 90% if the reaction is completed 50% in 15 minutes.
- MHT CET - 2024
- Chemistry
- kinetics equations
- IUPAC Name of Glyceraldehyde is:
- MHT CET - 2024
- Chemistry
- kinetics equations
- IUPAC Name of Acetone is:
- MHT CET - 2024
- Chemistry
- kinetics equations
- If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
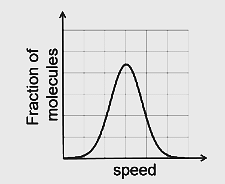
- JEE Advanced - 2020
- Chemistry
- kinetics equations
View More Questions
Questions Asked in KEAM exam
- Which among the following has the highest molar elevation constant?
- KEAM - 2025
- Colligative Properties
- The formula of Ammonium phosphomolybdate is
- KEAM - 2025
- coordination compounds
- Which is a Lewis acid?
- KEAM - 2025
- Acids and Bases
- Hardness of water is estimated by titration with
- KEAM - 2025
- Solutions
- Which of the following gases has the lowest solubility in water at 298 K?
- KEAM - 2025
- Solutions
View More Questions