Question:
If a 10 mM solution of a biomolecule in a cuvette of path length 10 mm absorbs 90% of the incident light at 280 nm, the molar extinction coefficient of the biomolecule at this wavelength is _______ M(^{-1})cm(^{-1}). (Round off to two decimal places)
If a 10 mM solution of a biomolecule in a cuvette of path length 10 mm absorbs 90% of the incident light at 280 nm, the molar extinction coefficient of the biomolecule at this wavelength is _______ M(^{-1})cm(^{-1}). (Round off to two decimal places)
Show Hint
When working with Beer-Lambert Law, ensure to convert all units properly; specifically, path length should be in centimeters for standard calculations.
Updated On: Feb 2, 2025
Hide Solution
Verified By Collegedunia
Solution and Explanation
To find the molar extinction coefficient, \( \epsilon \), we use the Beer-Lambert Law:
\[
A = \epsilon \cdot c \cdot l
\]
Where:
- \( A \) is the absorbance.
- \( \epsilon \) is the molar extinction coefficient.
- \( c \) is the concentration in molarity.
- \( l \) is the path length in centimeters.
Given that 90% of the incident light is absorbed, the absorbance \( A \) can be calculated using:
\[ A = -\log(1 - 0.90) = -\log(0.10) = 1 \] Step 2: Given Parameters.- Concentration: \( c = 10 \) mM = \( 0.01 \) M
- Path length: \( l = 10 \) mm = \( 1 \) cm
Therefore, the molar extinction coefficient is approximately \( 100 \, \text{M}^{-1} \text{cm}^{-1} \), with reasonable estimates between 98 and 102 \( \text{M}^{-1} \text{cm}^{-1} \) based on rounding and experimental considerations.
Was this answer helpful?
0
0
Top Questions on Mass spectrometry
- The major product in the given reaction sequence is Q. The mass spectrum of Q shows
([M] = molecular ion peak)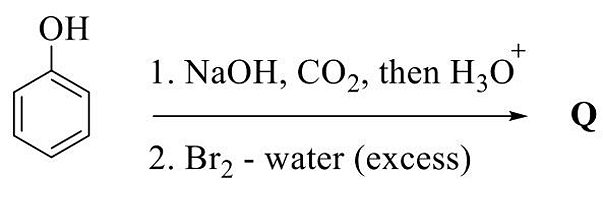
- GATE CY - 2024
- Inorganic Chemistry
- Mass spectrometry
- Which among the following characteristics of Laser light specifies the precise movement of all individual light waves together through time and space?
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Recombination of electron-hole produces ____ in LEDs.
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Magnetic sector analyzer is a part of
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which ionization technique in mass spectrometry is most suitable for large biomolecules like proteins:
- GPAT - 2024
- Pharmaceutical Analysis
- Mass spectrometry
View More Questions
Questions Asked in GATE XL exam
- Which enzyme is used to join two DNA fragments in genetic engineering?
- GATE XL - 2026
- Biotechnology
- Which pyramid is always upright in an ecosystem?
- The DNA double helix is stabilized primarily by:
- The process of conversion of nitrogen into ammonia by bacteria is called:
- GATE XL - 2026
- Microbiology
- Which of the following amino acids is essential in human diet?
- GATE XL - 2026
- Biochemistry
View More Questions