Question:
Recombination of electron-hole produces ____ in LEDs.
Recombination of electron-hole produces ____ in LEDs.
Show Hint
LED Operation. Forward bias \(\rightarrow\) Injection of electrons and holes \(\rightarrow\) Recombination in junction region \(\rightarrow\) Energy released as Photons (light). Light color depends on semiconductor bandgap energy.
Updated On: May 7, 2025
- Photons
- Protons
- Nucleus
- Junction capacitance
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
A Light Emitting Diode (LED) is a semiconductor device that emits light when forward biased
Under forward bias, electrons from the n-region and holes from the p-region are injected across the p-n junction
In the junction region (or active layer), these electrons and holes recombine
When an electron recombines with a hole, it transitions from a higher energy level (conduction band) to a lower energy level (valence band)
In direct bandgap semiconductors used for LEDs, this energy difference is released primarily in the form of a photon (a quantum of light)
The energy (and thus color) of the photon corresponds to the bandgap energy of the semiconductor material
Protons and nuclei are related to atomic structure, not light emission in LEDs
Junction capacitance is an electrical property of the diode
Under forward bias, electrons from the n-region and holes from the p-region are injected across the p-n junction
In the junction region (or active layer), these electrons and holes recombine
When an electron recombines with a hole, it transitions from a higher energy level (conduction band) to a lower energy level (valence band)
In direct bandgap semiconductors used for LEDs, this energy difference is released primarily in the form of a photon (a quantum of light)
The energy (and thus color) of the photon corresponds to the bandgap energy of the semiconductor material
Protons and nuclei are related to atomic structure, not light emission in LEDs
Junction capacitance is an electrical property of the diode
Was this answer helpful?
0
0
Top Questions on Mass spectrometry
- The major product in the given reaction sequence is Q. The mass spectrum of Q shows
([M] = molecular ion peak)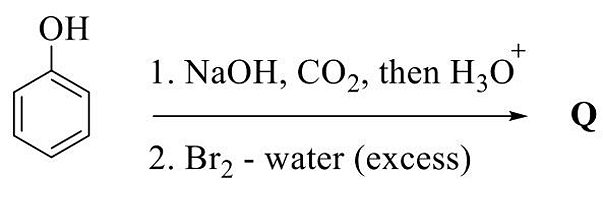
- GATE CY - 2024
- Inorganic Chemistry
- Mass spectrometry
- Which among the following characteristics of Laser light specifies the precise movement of all individual light waves together through time and space?
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Magnetic sector analyzer is a part of
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which ionization technique in mass spectrometry is most suitable for large biomolecules like proteins:
- GPAT - 2024
- Pharmaceutical Analysis
- Mass spectrometry
The spectrum of a protein obtained using electrospray ionization mass spectrometry (ESI-MS) is shown below. Two peaks, one at m/z = 2960.6 and the other at m/z = 3552.5, are marked. The mass of the protein associated with the m/z = 2960.6 peak is ______ Da. (Round off to two decimal places)

- GATE XL - 2024
- Biochemistry
- Mass spectrometry
View More Questions
Questions Asked in AP PGECET exam
- The absolute humidity of air at 101.325 kPa is measured to be 0.02 kg of water per kg of dry air. Then the partial pressure of water vapour in the air is:
- AP PGECET - 2025
- Thermodynamics
- If the vapour pressure at two temperatures of a solid phase in equilibrium with its liquid phase is known, then the latent heat of fusion can be calculated by the:
- AP PGECET - 2025
- Thermodynamics
- 1 mole of Argon gas is heated at constant pressure from 200 K to 600 K.
If \( C_p = 4\ \text{cal} \cdot \text{deg}^{-1} \cdot \text{mol}^{-1} \), then the change in entropy will be:
(Given \( \ln 3 = 1.09 \))
- AP PGECET - 2025
- Thermodynamics
- The minimum amount of work required to operate a refrigerator which removes 1000 Cal heat at $0^\circ$C and rejects at $50^\circ$C will be:
- AP PGECET - 2025
- Thermodynamics
- The entropy of single crystalline Silicon at absolute zero will be:
- AP PGECET - 2025
- Thermodynamics
View More Questions