Explain the mechanism of the cleaning action of soaps.
Explain the mechanism of the cleaning action of soaps.
Solution and Explanation
Cleansing action of soaps: The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the center of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.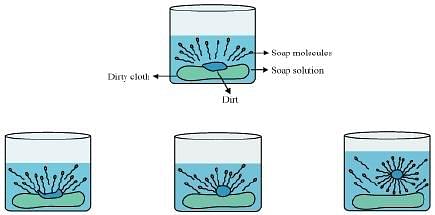
Top Questions on Chemical Properties Of Carbon Compounds
- (a) (i)Give reason why carbon can neither form C4+ cations nor C4− anions but form covalent compounds.
(ii) What is homologous series of carbon compound? Write the molecular formula of any two consecutive members of homologous series of aldehydes.
(iii) Draw the structure of the molecule of cyclohexane (C6H12).
OR
(b) (i) Name a commercially important carbon compound having functional group –OH and write its molecular formula. (ii) Write chemical equation to show its reaction with
(1) Sodium metal
(2) Excess conc. sulphuric acid
(3) Ethanoic acid in the presence of an acid catalyst
(4) Acidified potassium dichromate
Also write the name of the product formed in each case.- CBSE Class X - 2024
- Science
- Chemical Properties Of Carbon Compounds
- Consider the following statements about homologous series of carbon compounds:
(a) All succeeding members differ by –CH2 unit.
(b) Melting point and boiling point increases with increasing molecular mass.
(c) The difference in molecular masses between two successive members is 16 u.
(d) C2H2 and C3H4 are NOT the successive members of alkyne series.
The correct statements are –- CBSE Class X - 2024
- Science
- Chemical Properties Of Carbon Compounds
- Carbon compounds :
(i) are good conductors of electricity.
(ii) are bad conductors of electricity.
(iii) have strong forces of attraction between their molecules.
(iv) have weak forces of attraction between their molecules.
The correct statements are :- CBSE Class X - 2024
- Science
- Chemical Properties Of Carbon Compounds
- More than three million carbon compounds have been discovered in the field of chemistry. The diversity of these compounds is due to the capacity of carbon atoms for bonding with one another as well as with other atoms. Most of the carbon compounds are poor conductors of electricity and have low melting and boiling points.
- CBSE Class X - 2024
- Science
- Chemical Properties Of Carbon Compounds
Write notes on the following:
(a) Saturated and unsaturated hydrocarbons
(b) Corrosion
(c) Precipitation reaction- UP Board X - 2023
- Science
- Chemical Properties Of Carbon Compounds
Questions Asked in CBSE X exam
Leaves of the sensitive plant move very quickly in response to ‘touch’. How is this stimulus of touch communicated and explain how the movement takes place?
- CBSE Class X - 2025
- Plant Biology
- Rama is a farmer. She needs loan for agriculture work. Which of the following sources of loan will be beneficial for Rama? Choose the most appropriate option:
I. Bank
II. Agricultural Trader
III. Self-Help Group
IV. Government- CBSE Class X - 2025
- Money and Credit
Read the following sources of loan carefully and choose the correct option related to formal sources of credit:
(i) Commercial Bank
(ii) Landlords
(iii) Government
(iv) Money Lende- CBSE Class X - 2025
- Money and Credit
- Two statements are given below. They are Assertion (A) and Reason (R). Read both the statements carefully and choose the correct option. Assertion (A): Rupees is accepted as medium of exchange in India.
Reason (R): The World Bank legalises the use of rupee as a medium of payment in India.- CBSE Class X - 2025
- Money and Credit
- Rama is a farmer. She needs loan for agriculture work. Which of the following sources of loan will be beneficial for Rama? Choose the most appropriate option. I. Bank
II. Agricultural Trader
III. Self-Help Group
IV. Government- CBSE Class X - 2025
- Money and Credit
Concepts Used:
Allotropes of Carbon
The existence of a chemical element in one or more physical forms happening in the same physical state is called Allotropes or allotropy. Allotropes may show different physical or chemical states turning on the atom arrangement or the number of existent atoms. While carbon and sulfur is the common element presenting allotropy. Carbon can make further allotropes due to its five-membered valency.
Read More: Allotropes of Carbon
Allotropes of Carbon:
Carbon is part of a p-block element.
- The atomic number of carbon = 6
- It belongs to group 14.
- It makes up about 18.5% of the human body by mass.
Carbon has only two types of allotropes, such as;
- Crystalline
- Amorphous
The crystalline form of carbon consists of allotropes like;
- Diamond
- Graphite
- Fullerenes
The amorphous form of carbon consists of allotropes like;
- Coal
- Coke
- Wood charcoal
- Animal charcoal
- Lampblack
- Gas carbon