Question:
Which one of the following reactions does not occur during extraction of copper?
Which one of the following reactions does not occur during extraction of copper?
Updated On: Nov 20, 2025
- $CaO + SiO _2 \rightarrow CaSiO _3$
- $2 Cu _2 S +3 O _2 \rightarrow 2 Cu _2 O +2 SO _2$
- $FeO + SiO _2 \rightarrow FeSiO _3$
- $2 FeS +3 O _2 \rightarrow 2 FeO +2 SO _2$
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
No formation of calcium silicate in extraction of .
So, the correct option is (A): $CaO + SiO _2 \rightarrow CaSiO _3$
Was this answer helpful?
2
0
Top Questions on Thermodynamics terms
- Consider the following changes : $\text{SnO}_2 \rightarrow \text{SnO}$ $\Delta G_1^o$ and $\text{PbO}_2 \rightarrow \text{PbO}$ $\Delta G_2^o$. Select the correct option
- JEE Main - 2026
- Chemistry
- Thermodynamics terms
- One mole of a monatomic ideal gas is expanded by a process described by \( PV^3 = C \), where \( C \) is a constant. The heat capacity of the gas during the process is given by (R is the gas constant)
- OJEE - 2025
- Chemistry
- Thermodynamics terms
- One mole of a monatomic ideal gas is expanded by a process described by \( PV^3 = C \), where \( C \) is a constant. The heat capacity of the gas during the process is given by (R is the gas constant)
- OJEE - 2025
- Chemistry
- Thermodynamics terms
- The expression given below shows the variation of velocity \( v \) with time \( t \): \[ v = At^2 + \frac{Bt}{C + t}. \] The dimension of \( ABC \) is:
- JEE Main - 2025
- Physics
- Thermodynamics terms
- Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R. Assertion A: In a central force field, the work done is independent of the path chosen. Reason R: Every force encountered in mechanics does not have an associated potential energy. In the light of the above statements, choose the most appropriate answer from the options given below.
- JEE Main - 2025
- Physics
- Thermodynamics terms
View More Questions
Questions Asked in JEE Main exam
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
View More Questions
Concepts Used:
General Principles and Processes of Isolation of Elements
What are Ores and Minerals?
Minerals are the naturally occurring, homogeneous inorganic solid substances. They are having a definite chemical composition and crystalline structure, hardness and color. For example, copper pyrite, calamine, etc.
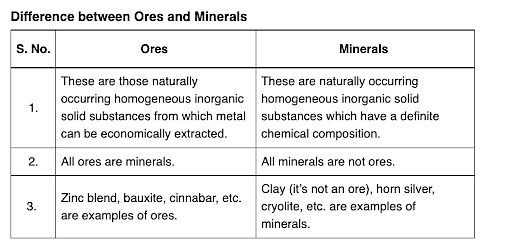
Impurities in an ore are called gauge. The removal of a gauge from the ore is called concentration ore.
Several steps are involved in the extraction of pure metal from ores. Major steps are as follows –
- Concentration of the ore
- Isolation of the metal from its concentrated ore
- Purification of the metal