Which one among the following metals is the weakest reducing agent?
- $Rb$
- $Na$
- $Li$
- $K$
The Correct Option is B
Approach Solution - 1
Sodium has the lowest oxidation potential in alkali metals. Hence it is the weakest reducing agent among alkali metals. Low ionization energy should be present for the element to act as a reducing agent. Alkali metals have low ionization energy thus making them a strong reducing agent.
So, the correct option is (B): Na.
Read more from the chapter: Classification of Elements and Periodicity in Properties
Approach Solution -2
The Correct Answer is (B)
Real Life Applications
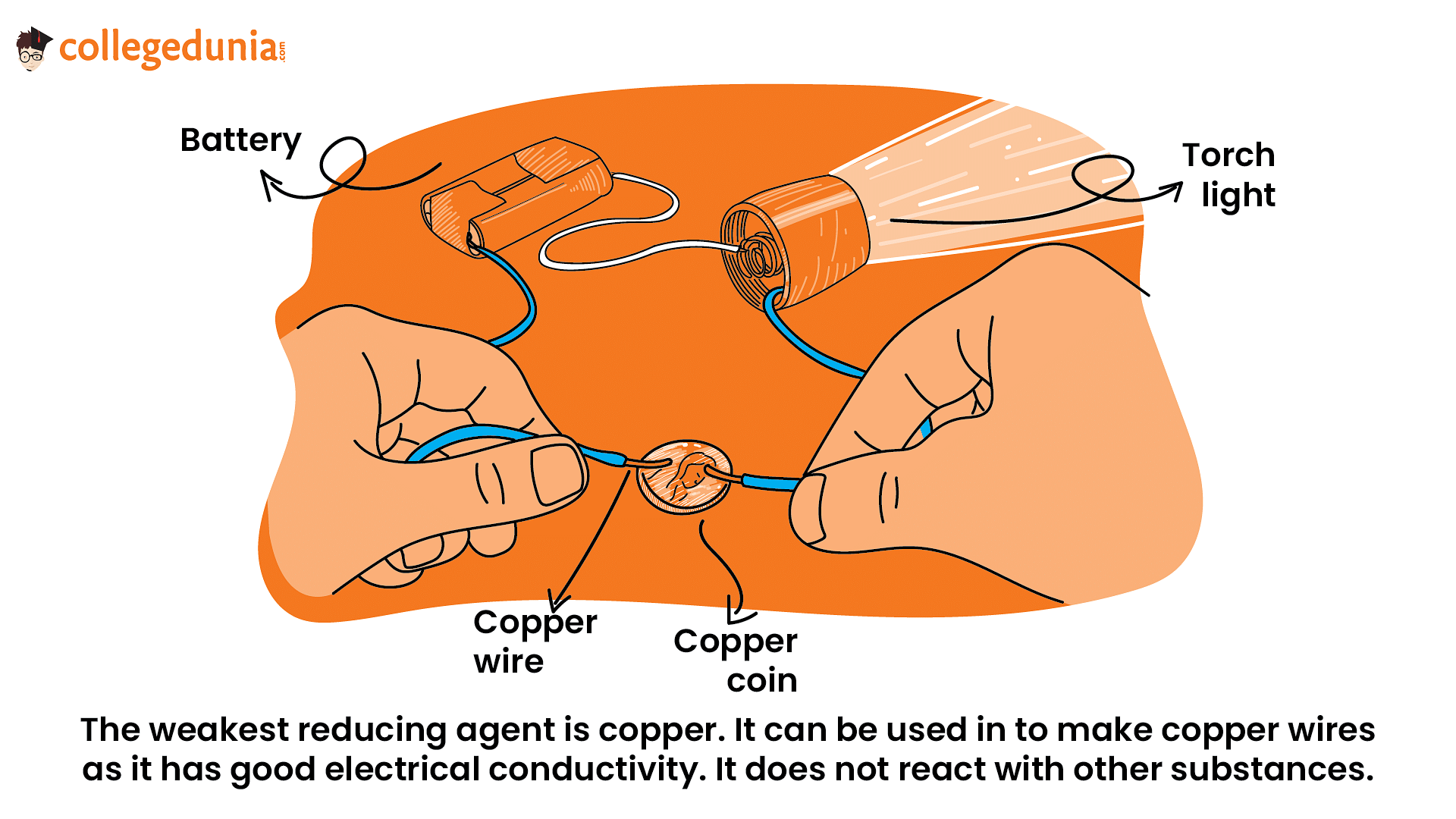
The weakest reducing agent is copper. It can be used in:
- To make copper wires as it has good electrical conductivity. It does not react with other substances.
- Copper coins are made from copper alloys that have zinc. It makes them durable.
- Copper is widely used to make marine applications as it will not react with seawater and thus is resistant to corrosion.
Question can also be asked as
- What is the weakest reducing agent among the following metals?
- How do the reducing abilities of these metals compare?
- Which metal is the most difficult to oxidize?
Approach Solution -3
The Correct Answer is (B)
Group 1 elements of the periodic table are known as Alkali metals. They are strong reducing agents. This is due to the single valence electron in the valence shell. The elements can easily lose their valence electron to acquire the noble gas configuration.
Reason for the alkali metal to act as a strong reducing agent.
Reducing agents: The elements that themselves get oxidized are called reducing agents. Due to the smaller nuclear charge and low ionization potential, they have a tendency to lose the electron easily. Thus making alkali metals strong reducing agents. As we go down from Li to Cs, the ionization potential decreases, and therefore the reducing power decreases. On the other hand, the hydration enthalpy of Sodium is the lowest thus making it the weakest reducing agent. Hydration enthalpy is the amount of energy released when one mole of ions is hydrated.
Read more
| Related Concepts | ||
|---|---|---|
| Redox reaction | Valence shell | Atom |
| Atomic mass of elements | Protons | Electrons |
Top Questions on p -Block Elements
Method used for separation of mixture of products (B and C) obtained in the following reaction is:

- JEE Main - 2026
- Chemistry
- p -Block Elements
- Statement-I: An element ‘X’ of P-block forms a hydride H–X, which has the longest bond length, then element ‘X’ will have the shortest covalent radius.
Statement-II: An element ‘E’ of Group 15 forms hydride EH$_3$, that has least B.P. The maximum covalency of E is 4.
- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements:
Statement I: [CoBr₄]²⁻ ion will absorb light of lower energy than [CoCl₄]²⁻ ion.
Statement II: In [CoBr₄]²⁻ ion, the energy separation between the two set of d-orbitals is more than [CoCl₄]²⁻ ion.
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements: Statement I: Elements \(X\) and \(Y\) are the most and least electronegative elements, respectively, among \(N\), \(As\), \(Sb\) and \(P\). The nature of the oxides \(X_2O_3\) and \(Y_2O_3\) is acidic and amphoteric, respectively. Statement II: \(BCl_3\) is covalent in nature and gets hydrolysed in water. It produces \([B(OH)_4]^-\) and \([B(H_2O)_6]^{3+}\) in aqueous medium. In the light of the above statements, choose the correct answer from the options given below:
- JEE Main - 2026
- Chemistry
- p -Block Elements
- For \( P \), the incorrect statement is:
- JEE Main - 2026
- Chemistry
- p -Block Elements
Questions Asked in JEE Main exam
- Let the circle \(x^2+y^2=4\) intersect the \(x\)-axis at points \(A(a,0)\) and \(B(b,0)\). Let \(P(2\cos\alpha,2\sin\alpha)\), \(0<\alpha<\frac{\pi}{2}\), and \(Q(2\cos\beta,2\sin\beta)\) be two points on the circle such that \((\alpha-\beta)=\frac{\pi}{2}\). Then the point of intersection of lines \(AQ\) and \(BP\) lies on:
- JEE Main - 2026
- Circles
- The moment of inertia of a square loop made of four uniform solid cylinders, each having radius R and length L (\(R \le L\)) about an axis passing through the mid points of opposite sides, is (Take the mass of the entire loop as M) :
- JEE Main - 2026
- Kinematics
- When a light of a given wavelength falls on a metallic surface the stopping potential for photoelectrons is \(3.2\ \text{V}\). If a second light having wavelength twice of the first light is used, the stopping potential drops to \(0.7\ \text{V}\). The wavelength of the first light is ________ m.
- JEE Main - 2026
- Photoelectric Effect
- An infinitely long straight wire carrying current $I$ is bent in a planar shape as shown in the diagram. The radius of the circular part is $r$. The magnetic field at the centre $O$ of the circular loop is :

- JEE Main - 2026
- Current electricity
- Identify the correct statements: The presence of –NO\(_2\) group in benzene ring A. activates the ring towards electrophilic substitutions. B. deactivates the ring towards electrophilic substitutions. C. activates the ring towards nucleophilic substitutions. D. deactivates the ring towards nucleophilic substitutions. Choose the correct answer from the options given below:
- JEE Main - 2026
- General Chemistry
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family