Which of the following has a square pyramidal shape?
PCI5
BrF5
PF5
[Ni(CN)4]2-
The Correct Option is B
Approach Solution - 1
\(BrF_5\) (Bromine pentafluoride) has a square pyramidal shape. This is because it has five regions of electron density around the central bromine atom, with one being a lone pair of electrons, resulting in a square pyramidal molecular geometry.
\(BrF_5\) has 1 lone pair and 5 bond. Therefore, geometry is octahedral, shape is square pyramidal.
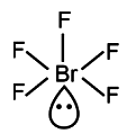
So, the correct option is (B): BrF5
Approach Solution -2
The question asks which molecule among the given options has a square pyramidal shape. To solve this, we need to understand the geometry of molecular structures based on VSEPR (Valence Shell Electron Pair Repulsion) theory.
- BrF5 (Bromine Pentafluoride):
- The central atom is bromine (Br), which is surrounded by 5 fluorine (F) atoms.
- Bromine has 7 valence electrons. It forms 5 bonds with fluorine atoms, utilizing 5 electrons.
- This leaves 2 electrons or 1 lone pair on the bromine atom.
- The electron pair geometry around bromine in BrF5 is octahedral (6 electron pairs counting bonds and lone pairs).
- The molecular shape, considering the lone pair, is square pyramidal.
- PCl5 (Phosphorus Pentachloride):
- Forms a trigonal bipyramidal shape due to 5 bond pairs and no lone pairs.
- PF5 (Phosphorus Pentafluoride):
- Similar to PCl5, it also has a trigonal bipyramidal shape with 5 bond pairs.
- [Ni(CN)4]2- (Tetracyanonickelate(II) ion):
- The shape of metal complexes like this involves coordination number and geometry.
- With a coordination number of 4, the geometry is typically square planar, not square pyramidal.
Thus, the correct answer is BrF5 which has a square pyramidal shape due to the presence of one lone pair on the bromine atom affecting the geometry. The lone pair occupies one vertex of the octahedron, resulting in a square pyramidal molecular shape.
Top Questions on Hybridisation
Arrange the following in increasing order of solubility product:
\[ {Ca(OH)}_2, {AgBr}, {PbS}, {HgS} \]- JEE Main - 2025
- Chemistry
- Hybridisation
Concentrated nitric acid is labelled as 75% by mass. The volume in mL of the solution which contains 30 g of nitric acid is:
Given: Density of nitric acid solution is 1.25 g/mL.- JEE Main - 2025
- Chemistry
- Hybridisation
Match List - I with List - II.
List - I (Saccharides) List - II (Glycosidic linkages found)
(A) Sucrose (I) \( \alpha 1 - 4 \)
(B) Maltose (II) \( \alpha 1 - 4 \) and \( \alpha 1 - 6 \)
(C) Lactose (III) \( \alpha 1 - \beta 2 \)
(D) Amylopectin (IV) \( \beta 1 - 4 \)Choose the correct answer from the options given below:
- JEE Main - 2025
- Chemistry
- Hybridisation
Match List - I with List - II.
List - I (Complex) List - II (Hybridisation) (A) \([\text{CoF}_6]^{3-}\) (I) \( d^2 sp^3 \) (B) \([\text{NiCl}_4]^{2-}\) (II) \( sp^3 \) (C) \([\text{Co(NH}_3)_6]^{3+}\) (III) \( sp^3 d^2 \) (D) \([\text{Ni(CN}_4]^{2-}\) (IV) \( dsp^2 \)
Choose the correct answer from the options given below:- JEE Main - 2025
- Chemistry
- Hybridisation
- The change in hybridisation (if any) of the 'Al' atom in the following reaction is
AlCl3 + Cl- → AlCl4-
- KCET - 2025
- Chemistry
- Hybridisation
Questions Asked in JEE Main exam
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
Concepts Used:
Hybridisation
Hybridization refers to the concept of combining atomic orbitals in order to form new hybrid orbitals that are appropriate to represent their bonding properties. Hybridization influences the bond length and bond strength in organic compounds.
Types of Hybridization:
sp Hybridization
sp hybridization is observed while one s and one p orbital inside the identical principal shell of an atom mix to shape two new equal orbitals. The new orbitals formed are referred to as sp hybridized orbitals.
sp2 Hybridization
sp2 hybridization is observed whilst ones and p orbitals of the same shell of an atom blend to shape three equivalent orbitals. The new orbitals formed are referred to as sp2 hybrid orbitals.
sp3 Hybridization
When one ‘s’ orbital and 3 ‘p’ orbitals belonging to the identical shell of an atom blend together to shape 4 new equal orbitals, the sort of hybridization is referred to as a tetrahedral hybridization or sp3.
sp3d Hybridization
sp3d hybridization involves the joining of 3p orbitals and 1d orbital to form 5 sp3d hybridized orbitals of identical energy. They possess trigonal bipyramidal geometry.
sp3d2 Hybridization
With 1 s three p’s and two d’s, there is a formation of 6 new and identical sp3d2 orbitals.