Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:- K and S
- Ca and O
- Al and N.
- K and S
- Ca and O
- Al and N.
Solution and Explanation
(a) \(K\) and \(S\):
The electronic configurations of \(K\) and \(S\) are as follows:
\(K\) : \(2, 8, 8, 1\)
\(S\) : \(2, 8, 6\)

Sulphur \((S)\) requires \(2\) more electrons to complete its octet. Potassium \((K)\) requires one electron more than the nearest noble gas i.e., \(Argon\). Hence, the electron transfer can be shown as:
(b) \(Ca\) and \(O\):
The electronic configurations of \(Ca\) and \(O\) are as follows:
\(Ca\) : \(2, 8, 8, 2\)
\(O\) : \(2, 6\)
Oxygen requires two electrons more to complete its octet, whereas calcium has two electrons more than the nearest noble gas i.e., \(Argon\). Hence, the electron transfer takes place as:
(c) \(Al\) and \(N\): The electronic configurations of \(Al\) and \(N\) are as follows:
\(Al\): \(2, 8, 3\)
\(N\) : \(2, 5\)
Nitrogen is three electrons short of the nearest noble gas (Neon), whereas aluminium has three electrons more than Neon.
Hence, the electron transference can be shown as:
Top Questions on Kossel-Lewis Approach to Chemical Bonding
- Which one follow 18 electron octet rule:
- JIPMER MBBS - 2019
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- Write the resonance structures for SO3 , NO2 and NO3-
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
Draw the Lewis structures for the following molecules and ions: \(H_2S\), \(SiCl_4\), \(BeF_2\), \(CO_3^{2-}\) , \(HCOOH\)
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- Define octet rule. Write its significance and limitations.
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
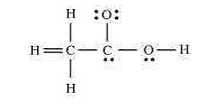
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
Questions Asked in CBSE Class XI exam
- Balance the following redox reactions by ion-electron method:
(a)MnO4- (aq) + I - (aq) → MnO2(s) + I2(s) (in basic medium)
(b) MnO4- (aq) + SO2(g) → Mn2+(aq) +HSO4- (aq) (in acidic solution)
(c) H2O2(aq)+Fe2+(aq) → Fe3+ (aq) + H2O (l) (in acidic solution)
(d) Cr2O72-+ SO2(g) → Cr3+ (aq) +SO42- (aq) (in acidic solution)- CBSE Class XI
- Oxidation Number
- At 700 K, equilibrium constant for the reaction:
\(H_2 (g) + I_2 (g) ⇋ 2HI (g)\)
is 54.8. If 0.5 mol L–1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700 K?- CBSE Class XI
- Law Of Chemical Equilibrium And Equilibrium Constant
- Find the mean deviation about the mean for the data 4, 7, 8, 9, 10, 12, 13, 17.
- CBSE Class XI
- Statistics
Find the mean deviation about the mean for the data 38, 70, 48, 40, 42, 55, 63, 46, 54, 44.
- CBSE Class XI
- Statistics
- Find the mean deviation about the median for the data.13, 17, 16, 14, 11, 13, 10, 16, 11, 18, 12, 17.
- CBSE Class XI
- Statistics