Define octet rule. Write its significance and limitations.
Solution and Explanation
The octet rule or the electronic theory of chemical bonding was developed by Kossel and Lewis. According to this rule, atoms can combine either by transfer of valence electrons from one atom to another or by sharing their valence electrons in order to attain the nearest noble gas configuration by having an octet in their valence shell.
The octet rule successfully explained the formation of chemical bonds depending upon the nature of the element.
Limitations of the octet theory:
The following are the limitations of the octet rule:
(a) The rule failed to predict the shape and relative stability of molecules.
(b) It is based upon the inert nature of noble gases. However, some noble gases like xenon and krypton form compounds such as \(XeF_2\), \(KrF_2\)etc.
(c) The octet rule cannot be applied to the elements in and beyond the third period of the periodic table. The elements present in these periods have more than eight valence electrons around the central atom. For example: \(PF_5\), \(SF_6\), etc.
(d) The octet rule is not satisfied for all atoms in a molecule having an odd number of electrons. For example, \(NO\) and \(NO_2\) do not satisfy the octet rule.
(e) This rule cannot be applied to those compounds in which the number of electrons surrounding the central atom is less than eight. For example, \(LiCl\), \(BeH_2\), \(AlCl_3\) etc. do not obey the octet rule.
Top Questions on Kossel-Lewis Approach to Chemical Bonding
- Which one follow 18 electron octet rule:
- JIPMER MBBS - 2019
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- Write the resonance structures for SO3 , NO2 and NO3-
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
Draw the Lewis structures for the following molecules and ions: \(H_2S\), \(SiCl_4\), \(BeF_2\), \(CO_3^{2-}\) , \(HCOOH\)
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- Use Lewis symbols to show electron transfer between the following atoms to form cations and anions:
- K and S
- Ca and O
- Al and N.
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
- The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
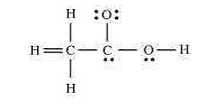
- CBSE Class XI
- Chemistry
- Kossel-Lewis Approach to Chemical Bonding
Questions Asked in CBSE Class XI exam
- Balance the following redox reactions by ion-electron method:
(a)MnO4- (aq) + I - (aq) → MnO2(s) + I2(s) (in basic medium)
(b) MnO4- (aq) + SO2(g) → Mn2+(aq) +HSO4- (aq) (in acidic solution)
(c) H2O2(aq)+Fe2+(aq) → Fe3+ (aq) + H2O (l) (in acidic solution)
(d) Cr2O72-+ SO2(g) → Cr3+ (aq) +SO42- (aq) (in acidic solution)- CBSE Class XI
- Oxidation Number
- At 700 K, equilibrium constant for the reaction:
\(H_2 (g) + I_2 (g) ⇋ 2HI (g)\)
is 54.8. If 0.5 mol L–1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700 K?- CBSE Class XI
- Law Of Chemical Equilibrium And Equilibrium Constant
- Find the mean deviation about the mean for the data 4, 7, 8, 9, 10, 12, 13, 17.
- CBSE Class XI
- Statistics
Find the mean deviation about the mean for the data 38, 70, 48, 40, 42, 55, 63, 46, 54, 44.
- CBSE Class XI
- Statistics
- Find the mean deviation about the median for the data.13, 17, 16, 14, 11, 13, 10, 16, 11, 18, 12, 17.
- CBSE Class XI
- Statistics