Transduction in bacteria was discovered by
- Zinder and Lederberg
- Herelle and Twort
- Wallace and Jacob
- Lederberg and Tatum
The Correct Option is A
Solution and Explanation
Top Questions on biological classification
- Which of the following microorganisms is used in the production of curd from milk?
- MHT CET - 2025
- Biology
- biological classification
- In a DNA molecule, which of the following base-pairings is correct?
- MHT CET - 2025
- Biology
- biological classification
- Which is not a prime element?
- MHT CET - 2025
- Biology
- biological classification
- Study the following and choose the incorrect combinations:
I. Phylum: Porifera, Special cells: Lasso cells, Example: Spongilla
II. Phylum: Cnidaria, Special cells: Stinging cells, Example: Hydra
III. Phylum: Ctenophora, Special cells: Choanocytes, Example: Pleurobrachia
IV. Phylum: Platyhelminthes, Special cells: Flame cells, Example: Fasciola- TS EAMCET - 2025
- Zoology
- biological classification
- Study the following and choose the correct combinations:

- TS EAMCET - 2025
- Zoology
- biological classification
Questions Asked in COMEDK UGET exam
- Given that the freezing point of benzene is $ 5.48^\circ C $ and its $ K_f $ value is $ 5.12^\circ C/m $, what would be the freezing point of a solution of 20 g of propane in 400 g of benzene?
- COMEDK UGET - 2024
- Colligative Properties
200 ml of an aqueous solution contains 3.6 g of Glucose and 1.2 g of Urea maintained at a temperature equal to 27$^{\circ}$C. What is the Osmotic pressure of the solution in atmosphere units?
Given Data R = 0.082 L atm K$^{-1}$ mol$^{-1}$
Molecular Formula: Glucose = C$_6$H$_{12}$O$_6$, Urea = NH$_2$CONH$_2$- COMEDK UGET - 2024
- Colligative Properties
- An inorganic compound W undergoes the following reactions: $ W + \text{Na}_2\text{CO}_3 \xrightarrow{\text{O}_2 / \text{heat}} X + H^+ \xrightarrow{} Y(s) $ $ Y(aq) + \text{KCl} (aq) \xrightarrow{} Z(s) $ Z appears in the form of orange crystals and is used as an oxidising agent in acid medium. Identify the compound W.
- COMEDK UGET - 2024
- coordination compounds
- A current of 3.0 A is passed through 750 ml of 0.45 M solution of CuSO₄ for 2 hours with a current efficiency of 90\%. If the volume of the solution is assumed to remain constant, what would be the final molarity of CuSO₄ solution?
- COMEDK UGET - 2024
- Solutions
- For a reaction $ 5X + Y \to 3Z $, the rate of formation of Z is $ 2.4 \times 10^{-5} \, \text{mol L}^{-1} \text{s}^{-1} $. Calculate the average rate of disappearance of X.
- COMEDK UGET - 2024
- Stoichiometry and Stoichiometric Calculations
Concepts Used:
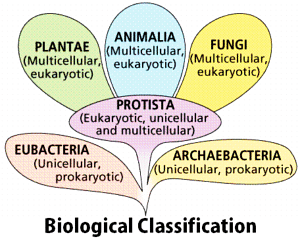
Biological Classification
The process of grouping living organisms into categories is called biological classification. The most modern 5-kingdom classification was put ahead by an eminent scientist R.H.Whittaker. The five-kingdom classification is based on the criteria like cell structure, mode of nutrition, body form, and reproduction. One of the most important characteristics of this system is that it follows the evolutionary sequence of living organisms. The organisms are classified into distinct taxa or levels like Kingdom, Phylum, Division, Class, Order, Family, Genus, and Species. The 5 kingdoms are as follows: