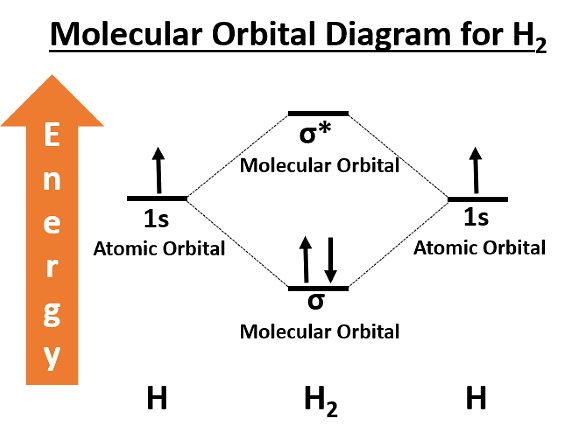
The wave functions of $1s$ orbitals of two hydrogen atoms are $\Psi_A$ and $\Psi_B$. $\Psi_A$ and $\Psi_B$ are linearly combined to form two molecular orbitals ($\sigma$ and $\sigma^{\ast}$). Which of the following statements are collect?
I. $\sigma^{*}$ is equal to $(\Psi_{A}-\Psi_{B})$.
II. In $\sigma$ - orbital, one nodal plane is present in between two nuclei.
III. The energy of $\sigma$ - orbital is lower than the energy of $\sigma^{*}$ -orbital.
- I ,II ,III
- I, II only
- II, III only
- I, III only
The Correct Option is D
Solution and Explanation
I. $\sigma^{*}$ -orbital are formed by the substraction of two wave functions say $\psi_{A}$ and $\psi_{B}$ and therefore $\sigma^{*}=\psi_{A}-\psi_{B}$
II. $\sigma$-orbital are formed when two orbitals are in same phase and thus, do not have nodal plane in between two nuclei.
III. Combination of two sigma $(\sigma)$ atomic orbitals given two molecular orbitals, out of which one is of lower energy called $\sigma$ bonding orbital and other is of higher energy called $\sigma^{*}$ anti-bonding orbital.
Hence, (i) and (iii) are the correct statements
Top Questions on Molecular Orbital Theory
- Pair of species among the following having same bond order as well as paramagnetic character will be:
- JEE Main - 2026
- Chemistry
- Molecular Orbital Theory
- Among the species O$_2^+$, N$_2^-$, N$_2^{2-}$ and O$_2^-$ which have same bond order as well as paramagnetic in nature.
- JEE Main - 2026
- Chemistry
- Molecular Orbital Theory
Regarding the molecular orbital (MO) energy levels for homonuclear diatomic molecules, the INCORRECT statement(s) is (are):
- JEE Advanced - 2025
- Chemistry
- Molecular Orbital Theory
- Arrange the following in increasing order of bond order: (A) He\(_2^+\)
(B) O\(_2^-\)
(C) HF
(D) NO\(^-\)- CUET (PG) - 2025
- Chemistry
- Molecular Orbital Theory
- Which of the following is the ratio of 5\(^\text{th}\) Bohr orbit \( (r_5) \) of He\(^+\) & Li\(^{2+}\)?
- JEE Main - 2025
- Chemistry
- Molecular Orbital Theory
Questions Asked in AP EAMCET exam
Which of the following are ambident nucleophiles?
[A.] CN$^{\,-}$
[B.] CH$_{3}$COO$^{\,-}$
[C.] NO$_{2}^{\,-}$
[D.] CH$_{3}$O$^{\,-}$
[E.] NH$_{3}$- AP EAMCET - 2024
- Acids and Bases
- The correct sequence of enzymes involved in the commercial production of ethanol by fermentation from sugar is:
- AP EAMCET - 2024
- Acids and Bases
- At 298 K, the ionization constant of \( {CN}^- \) is \( 2.08 \times 10^{-6} \). What is the ionization constant of its conjugate acid? (Given \( K_w = 10^{-14} \))
- AP EAMCET - 2024
- Acids and Bases
Identify the anomers from the following.

- AP EAMCET - 2024
- Acids and Bases
The standard Gibbs free energy change \( \Delta G^\circ \) of a cell reaction is \(-301 { kJ/mol}\). What is \( E^\circ \) in volts?
(Given: \( F = 96500 { C/mol}\), \( n = 2 \))- AP EAMCET - 2024
- Acids and Bases
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.