The topicity relationship of \(H_a\) and \(H_b\) in X, Y and Z (as drawn in the figure) are, respectively,

The topicity relationship of \(H_a\) and \(H_b\) in X, Y and Z (as drawn in the figure) are, respectively, 
Show Hint
- Diastereotopic, Homotopic and Enantiotopic
- Homotopic, Enantiotopic and Enantiotopic
- Homotopic, Homotopic and Enantiotopic
- Diastereotopic, Enantiotopic and Homotopic
The Correct Option is B
Solution and Explanation
Step 1: Molecule X. The two hydrogens \(H_a\) and \(H_b\) lie on a carbon in a highly symmetric (meso/C\(_2\)) environment bearing identical HO− and −CO2H substituent sets on opposite sides. A \(C_2\) rotation superposes \(H_a\) and \(H_b\); replacing either gives the same molecule \(⇒\) homotopic.
Step 2: Molecule Y. In the bridged/aromatic framework, \(H_a\) and \(H_b\) are related by an improper/mirror operation: replacement of \(H_a\) versus \(H_b\) generates non-superposable mirror-image products, while the parent is achiral. Thus they are enantiotopic.
Step 3: Molecule Z. For the prochiral vinyl \({CH_2}\) adjacent to −Cl, the two vinylic hydrogens are in an enantiotopic relationship (replacing one or the other creates enantiomeric \(E/R_e\) vs \(S_i\) labeled products). Hence enantiotopic.
Step 4: Collecting: \(X\) homotopic, \(Y\) enantiotopic, \(Z\) enantiotopic \(⇒\) option (B).
Top Questions on Relative stereochemistry in compounds having more than one stereogenic centre
- Fischer presentation of D-(-)-fructose is given below.
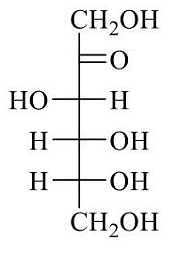
The correct structure of α-L-(+)-fructofuranose is- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The major products X and Y in the following reaction sequence are
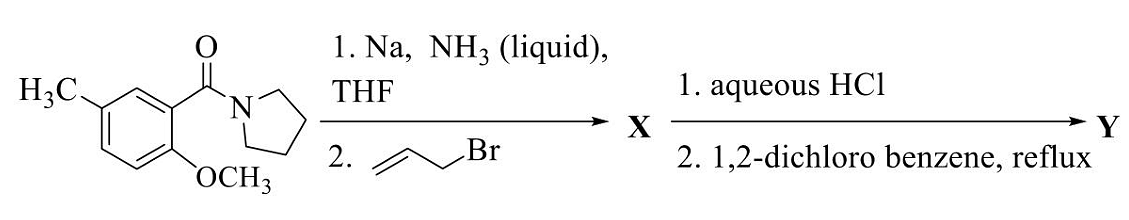
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The correct statement(s) for decalin is (are)
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The reaction(s) in which inversion of configuration occur(s) is(are)

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The acetolysis product(s) of the given reaction (solvolysis of the benzylic tosylate in AcOH) is(are)

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
Questions Asked in GATE CY exam
- For a first-order reaction, the unit of rate constant is:
- GATE CY - 2026
- Chemical Kinetics
- Which ligand causes maximum crystal field splitting?
- GATE CY - 2026
- Coordination chemistry
- The standard electrode potential of the standard hydrogen electrode (SHE) is:
- GATE CY - 2026
- Electrochemistry
- Which of the following reagents converts an aldehyde selectively into a primary alcohol?
- GATE CY - 2026
- Organic Chemistry
- In IR spectroscopy, which bond absorbs at the highest wavenumber?
- GATE CY - 2026
- Spectroscopy