Question:
The reaction(s) in which inversion of configuration occur(s) is(are)

The reaction(s) in which inversion of configuration occur(s) is(are) 
Show Hint
{Mitsunobu} \(=\) activated alcohol \(\mathrm{S_N2}\) \(⇒\) inversion.
{Neighboring-group participation} (acyloxonium/thiiranium/halonium) often leads to backside opening \(⇒\) inversion at the attacked carbon.
{Rearrangements} like Hofmann and Baeyer–Villiger are concerted migrations \(⇒\) {retention} of configuration at the migrating center.
{Neighboring-group participation} (acyloxonium/thiiranium/halonium) often leads to backside opening \(⇒\) inversion at the attacked carbon.
{Rearrangements} like Hofmann and Baeyer–Villiger are concerted migrations \(⇒\) {retention} of configuration at the migrating center.
Updated On: Aug 29, 2025
Hide Solution
Verified By Collegedunia
Solution and Explanation
Step 1: Option (A) — Mitsunobu reaction.
Ph3P/DEAD with a carboxylic acid converts an alcohol R*–OH into the corresponding ester R*–O2CR' via an SN2 displacement of the O–PPh3 activated intermediate. The chiral center at carbon undergoes a single backside attack ⇒ Walden inversion.
Step 2: Option (B) — Anchimeric assistance (acyloxonium) under heat.
On heating, the neighboring acetate participates to form a cyclic acyloxonium intermediate; subsequent intramolecular/backside attack (or acetate capture) occurs SN2-like. The carbon bearing the leaving group is attacked from the opposite face ⇒ inversion at that stereocenter. (This is the classic neighboring-group participation in constrained bicyclic/allylic systems.)
Step 3: Option (C) — Hofmann rearrangement.
NaOBr converts an amide to a C-1 shorter amine via N-bromoamide, rearrangement, and isocyanate. Stereochemical information at a migrating alpha-carbon (if present) is retained through the concerted migration; no inversion is involved.
Step 4: Option (D) — Baeyer–Villiger oxidation.
Peracid inserts an oxygen adjacent to a carbonyl with migration of one substituent. The migrating stereocenter (if any) migrates with retention (concerted rearrangement), not inversion.
Therefore, only (A) and (B) involve inversion of configuration.
Ph3P/DEAD with a carboxylic acid converts an alcohol R*–OH into the corresponding ester R*–O2CR' via an SN2 displacement of the O–PPh3 activated intermediate. The chiral center at carbon undergoes a single backside attack ⇒ Walden inversion.
Step 2: Option (B) — Anchimeric assistance (acyloxonium) under heat.
On heating, the neighboring acetate participates to form a cyclic acyloxonium intermediate; subsequent intramolecular/backside attack (or acetate capture) occurs SN2-like. The carbon bearing the leaving group is attacked from the opposite face ⇒ inversion at that stereocenter. (This is the classic neighboring-group participation in constrained bicyclic/allylic systems.)
Step 3: Option (C) — Hofmann rearrangement.
NaOBr converts an amide to a C-1 shorter amine via N-bromoamide, rearrangement, and isocyanate. Stereochemical information at a migrating alpha-carbon (if present) is retained through the concerted migration; no inversion is involved.
Step 4: Option (D) — Baeyer–Villiger oxidation.
Peracid inserts an oxygen adjacent to a carbonyl with migration of one substituent. The migrating stereocenter (if any) migrates with retention (concerted rearrangement), not inversion.
Therefore, only (A) and (B) involve inversion of configuration.
Was this answer helpful?
0
0
Top Questions on Relative stereochemistry in compounds having more than one stereogenic centre
- Fischer presentation of D-(-)-fructose is given below.
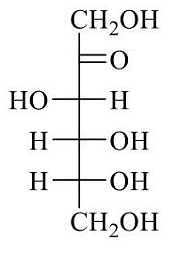
The correct structure of α-L-(+)-fructofuranose is- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The major products X and Y in the following reaction sequence are
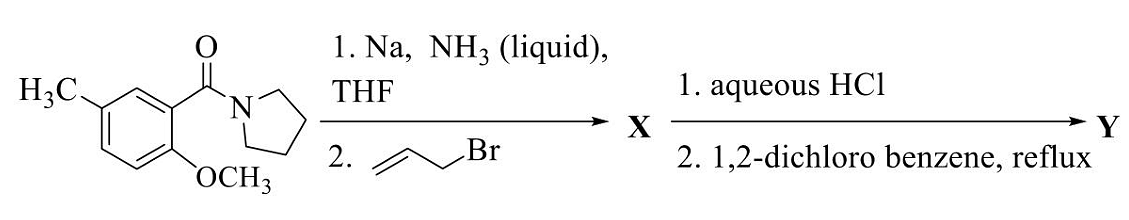
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The correct statement(s) for decalin is (are)
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The acetolysis product(s) of the given reaction (solvolysis of the benzylic tosylate in AcOH) is(are)

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The topicity relationship of \(H_a\) and \(H_b\) in X, Y and Z (as drawn in the figure) are, respectively,

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
Questions Asked in GATE CY exam
- For a first-order reaction, the unit of rate constant is:
- GATE CY - 2026
- Chemical Kinetics
- Which ligand causes maximum crystal field splitting?
- GATE CY - 2026
- Coordination chemistry
- The standard electrode potential of the standard hydrogen electrode (SHE) is:
- GATE CY - 2026
- Electrochemistry
- Which of the following reagents converts an aldehyde selectively into a primary alcohol?
- GATE CY - 2026
- Organic Chemistry
- In IR spectroscopy, which bond absorbs at the highest wavenumber?
- GATE CY - 2026
- Spectroscopy
View More Questions