Question:
The acetolysis product(s) of the given reaction (solvolysis of the benzylic tosylate in AcOH) is(are)

The acetolysis product(s) of the given reaction (solvolysis of the benzylic tosylate in AcOH) is(are) 
Show Hint
Benzylic solvolysis next to an aryl group often proceeds via a {phenonium} ion; nucleophiles open the bridge {anti}, giving net inversion at that carbon.
Stereocenters not involved in bond making/breaking retain configuration.
When options show pairs, look for “inversion vs retention” at the reacting center to decide.
Updated On: Aug 29, 2025
Hide Solution
Verified By Collegedunia
Solution and Explanation
Step 1: Ionization and anchimeric assistance.
In \( \mathrm{AcOH} \), the benzylic tosylate undergoes solvolysis. The departure of \( \mathrm{OTs}^- \) generates a benzylic cation. This benzylic cation is then stabilized by a neighboring group, in this case, the adjacent phenyl ring, which provides anchimeric assistance. This neighboring-group participation shields one face of the cationic center, resulting in a unique intermediate. The cation formed is not planar, as the adjacent phenyl group plays a significant role in stabilizing the cation by forming a bridged phenonium intermediate. This mechanism allows the system to minimize the energetic instability of the benzylic cation.
Step 2: Regio-/stereochemical capture.
The nucleophilic attack by \( \mathrm{AcO}^- \) occurs via an anti opening of the phenonium bridge at the reacting carbon. The anti opening is a key factor here because it results in an inversion of configuration at the substitution center. This means that the attack occurs from the opposite side of the leaving group, similar to an SN2 reaction mechanism. This leads to an overall inversion at the reacting center. Importantly, the other stereocenter, which is not directly involved in the reaction (since its bonds remain unbroken), retains its original configuration. This is a classic feature of reactions involving anti opening and inversion at the center undergoing substitution.
Step 3: Products formed.
As a result of the acetolysis, two diastereomers are formed. Both of these diastereomers exhibit inversion at the reacting center, which occurs due to the attack from the two accessible conformers formed during the anti opening of the phenonium intermediate. Therefore, options (A) and (C) are the correct products because they reflect the inversion of configuration at the reacting center, consistent with the described mechanism. On the other hand, options (B) and (D) depict retention of configuration at the reacting center, which is not favored in this case due to the anchimeric assistance. The neighboring-group participation leads to an anti attack and inversion, thus making options (B) and (D) incorrect.
Final Answer: \( \boxed{\text{Anchimeric assistance by Ph } \Rightarrow \text{ anti attack and inversion at the benzylic center } \Rightarrow \text{ (A) and (C).}} \)
In \( \mathrm{AcOH} \), the benzylic tosylate undergoes solvolysis. The departure of \( \mathrm{OTs}^- \) generates a benzylic cation. This benzylic cation is then stabilized by a neighboring group, in this case, the adjacent phenyl ring, which provides anchimeric assistance. This neighboring-group participation shields one face of the cationic center, resulting in a unique intermediate. The cation formed is not planar, as the adjacent phenyl group plays a significant role in stabilizing the cation by forming a bridged phenonium intermediate. This mechanism allows the system to minimize the energetic instability of the benzylic cation.
Step 2: Regio-/stereochemical capture.
The nucleophilic attack by \( \mathrm{AcO}^- \) occurs via an anti opening of the phenonium bridge at the reacting carbon. The anti opening is a key factor here because it results in an inversion of configuration at the substitution center. This means that the attack occurs from the opposite side of the leaving group, similar to an SN2 reaction mechanism. This leads to an overall inversion at the reacting center. Importantly, the other stereocenter, which is not directly involved in the reaction (since its bonds remain unbroken), retains its original configuration. This is a classic feature of reactions involving anti opening and inversion at the center undergoing substitution.
Step 3: Products formed.
As a result of the acetolysis, two diastereomers are formed. Both of these diastereomers exhibit inversion at the reacting center, which occurs due to the attack from the two accessible conformers formed during the anti opening of the phenonium intermediate. Therefore, options (A) and (C) are the correct products because they reflect the inversion of configuration at the reacting center, consistent with the described mechanism. On the other hand, options (B) and (D) depict retention of configuration at the reacting center, which is not favored in this case due to the anchimeric assistance. The neighboring-group participation leads to an anti attack and inversion, thus making options (B) and (D) incorrect.
Final Answer: \( \boxed{\text{Anchimeric assistance by Ph } \Rightarrow \text{ anti attack and inversion at the benzylic center } \Rightarrow \text{ (A) and (C).}} \)
Was this answer helpful?
0
0
Top Questions on Relative stereochemistry in compounds having more than one stereogenic centre
- Fischer presentation of D-(-)-fructose is given below.
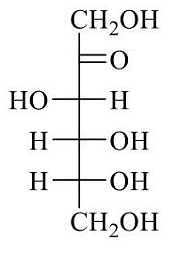
The correct structure of α-L-(+)-fructofuranose is- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The major products X and Y in the following reaction sequence are
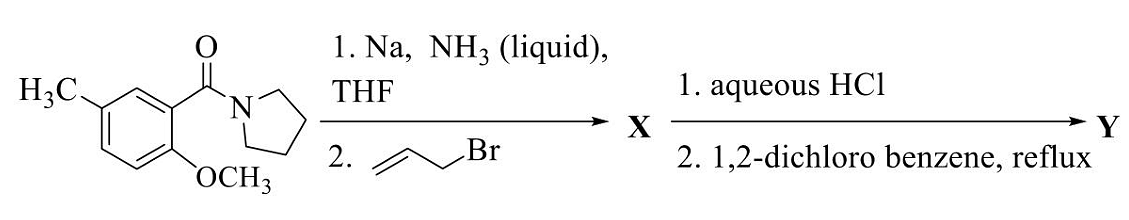
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
- The correct statement(s) for decalin is (are)
- GATE CY - 2024
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The reaction(s) in which inversion of configuration occur(s) is(are)

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
The topicity relationship of \(H_a\) and \(H_b\) in X, Y and Z (as drawn in the figure) are, respectively,

- GATE CY - 2023
- Organic Chemistry
- Relative stereochemistry in compounds having more than one stereogenic centre
Questions Asked in GATE CY exam
- For a first-order reaction, the unit of rate constant is:
- GATE CY - 2026
- Chemical Kinetics
- Which ligand causes maximum crystal field splitting?
- GATE CY - 2026
- Coordination chemistry
- The standard electrode potential of the standard hydrogen electrode (SHE) is:
- GATE CY - 2026
- Electrochemistry
- Which of the following reagents converts an aldehyde selectively into a primary alcohol?
- GATE CY - 2026
- Organic Chemistry
- In IR spectroscopy, which bond absorbs at the highest wavenumber?
- GATE CY - 2026
- Spectroscopy
View More Questions