Question:
The magnetic moment of the complex species- [Fe(H2O)6]2- and K2[MnCl4] are 5.3 and 5.9 respectively. The number of unpaired electrons respectively are :
The magnetic moment of the complex species- [Fe(H2O)6]2- and K2[MnCl4] are 5.3 and 5.9 respectively. The number of unpaired electrons respectively are :
Updated On: Mar 27, 2025
- 4, 5
- 4, 4
- 5, 5
- 5, 4
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The correct option is (A) :4, 5.
Was this answer helpful?
0
0
Top Questions on The Magnetic Dipole Moment
- Arrange the following solids in the decreasing order of their magnetic strength in the presence of external magnetic field.
A. NaCl
B. CrO2
C. MnO
D. Fe3O4- CUET (UG) - 2023
- Chemistry
- The Magnetic Dipole Moment
- Match List I with List IIChoose the correct answer from the options given below:
LIST I LIST II A. Magnetic dipole moment I. Weber/$m^2$ B. Magnetic permeability II. Am (Ampere m) C. Pole strength III. Henry/m D. Magnetic flux density IV. $Am^2$ (Ampere $m^2$) - CUET (UG) - 2023
- Physics
- The Magnetic Dipole Moment
- The magnetic moment of a trivalent ion of a metal with Z = 24 in aqueous solution is
- KEAM - 2021
- Chemistry
- The Magnetic Dipole Moment
- Following figures show the arrangement of bar magnets in different configurations. Each magnet has magnetic dipole moment \(\overrightarrow{m}\). Which configuration has highest net magnètic dipole moment?
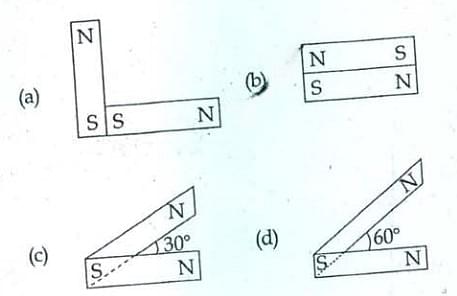
- NEET (UG) - 2014
- Physics
- The Magnetic Dipole Moment
- A bar magnet of length I and magnetic dipole moment M is bent in the form of an arc as shown in figure. The new magnetic dipole moment will be
- NEET (UG) - 2013
- Physics
- The Magnetic Dipole Moment
Questions Asked in CUET exam
- Find the ratio of de-Broglie wavelengths of deuteron having energy E and \(\alpha\)-particle having energy 2E :
- CUET (UG) - 2026
- Dual nature of radiation and matter
- Match List-I with List-II \[ \begin{array}{|l|l|} \hline \textbf{Solutions} & \textbf{Explanation} \\ \hline (A) \; \text{Saturated solution} & (I) \; \text{Solution having two components.} \\ \hline (B) \; \text{Isotonic solutions} & (II) \; \text{A solution whose osmotic pressure is more than that of another.} \\ \hline (C) \; \text{Binary solution} & (III) \; \text{A solution which contains the maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature.} \\ \hline (D) \; \text{Hypertonic solution} & (IV) \; \text{The solutions having the same osmotic pressure at a given temperature.} \\ \hline \end{array} \]
- CUET (UG) - 2025
- General Chemistry
- The de-Broglie wavelength associated with a ball of mass 150 g traveling at 30.0 m/s would be
- CUET (UG) - 2025
- de broglie hypothesis
- A clock shows the time as 3:15. What is the angle between the hour and minute hands?
- CUET (UG) - 2025
- Clock and Calendar
- If 36: 84 :: 42: X, then the value of X, is:
- CUET (UG) - 2025
- Ratio and Proportion
View More Questions