Question:
The magnetic moment of a trivalent ion of a metal with Z = 24 in aqueous solution is
The magnetic moment of a trivalent ion of a metal with Z = 24 in aqueous solution is
Updated On: Apr 4, 2025
- 3.87 BM
- 2.84 BM
- 1.73 BM
- 4.90 BM
- 5.92 BM
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The magnetic moment (\(\mu\)) for a metal ion can be calculated using the formula:
\(\mu = \sqrt{n(n + 2)}\)
where \(n\) is the number of unpaired electrons. Now, for Z = 24, the electronic configuration of the element is:
Cr (Z = 24) → [Ar] 3d5 4s1
The trivalent ion (Cr3+) has an electronic configuration of:
Cr3+ → [Ar] 3d3
This indicates there are 3 unpaired electrons in the d-orbital. Hence, \(n = 3\).
Now, we substitute this value into the magnetic moment formula:
\(\mu = \sqrt{3(3 + 2)} = \sqrt{3 \times 5} = \sqrt{15} \approx 3.87 \, \text{BM}\)
This matches the value given in option (1), so the correct magnetic moment for Cr3+ is 3.87 BM.
Was this answer helpful?
0
0
Top Questions on The Magnetic Dipole Moment
- The magnetic moment of the complex species- [Fe(H2O)6]2- and K2[MnCl4] are 5.3 and 5.9 respectively. The number of unpaired electrons respectively are :
- CUET (UG) - 2023
- Chemistry
- The Magnetic Dipole Moment
- Arrange the following solids in the decreasing order of their magnetic strength in the presence of external magnetic field.
A. NaCl
B. CrO2
C. MnO
D. Fe3O4- CUET (UG) - 2023
- Chemistry
- The Magnetic Dipole Moment
- Match List I with List IIChoose the correct answer from the options given below:
LIST I LIST II A. Magnetic dipole moment I. Weber/$m^2$ B. Magnetic permeability II. Am (Ampere m) C. Pole strength III. Henry/m D. Magnetic flux density IV. $Am^2$ (Ampere $m^2$) - CUET (UG) - 2023
- Physics
- The Magnetic Dipole Moment
- Following figures show the arrangement of bar magnets in different configurations. Each magnet has magnetic dipole moment \(\overrightarrow{m}\). Which configuration has highest net magnètic dipole moment?
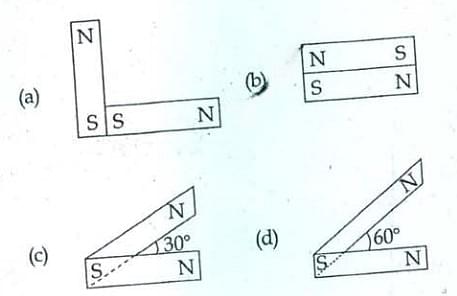
- NEET (UG) - 2014
- Physics
- The Magnetic Dipole Moment
- A bar magnet of length I and magnetic dipole moment M is bent in the form of an arc as shown in figure. The new magnetic dipole moment will be
- NEET (UG) - 2013
- Physics
- The Magnetic Dipole Moment
Questions Asked in KEAM exam
- Which among the following has the highest molar elevation constant?
- KEAM - 2025
- Colligative Properties
- The formula of Ammonium phosphomolybdate is
- KEAM - 2025
- coordination compounds
- Which is a Lewis acid?
- KEAM - 2025
- Acids and Bases
- Hardness of water is estimated by titration with
- KEAM - 2025
- Solutions
- Which of the following gases has the lowest solubility in water at 298 K?
- KEAM - 2025
- Solutions
View More Questions