Question:
The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301)
The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301)
Updated On: Nov 25, 2025
- 23.03 minutes
- 46.06minutes
- 460.6 minutes
- 230.03 minutes
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
For first order reaction,
$k=\frac{0.693}{t_{1 /2} }=\frac{0.693}{6.93}$
$k=\frac{2.303}{t} \, log\, \frac{100}{100-99}$
$\frac{0.693}{6.93}=\frac{2.303}{t} log\, \frac{100}{1}$
$\frac{0.693}{6.93}=\frac{2.303\times2}{t}$
t=46.06 min
$k=\frac{0.693}{t_{1 /2} }=\frac{0.693}{6.93}$
$k=\frac{2.303}{t} \, log\, \frac{100}{100-99}$
$\frac{0.693}{6.93}=\frac{2.303}{t} log\, \frac{100}{1}$
$\frac{0.693}{6.93}=\frac{2.303\times2}{t}$
t=46.06 min
Was this answer helpful?
0
0
Top Questions on kinetics equations
- Magnetic Moment of \( \text{Mn}^{2+} \) is:
- MHT CET - 2024
- Chemistry
- kinetics equations
Find the time required to complete a reaction 90% if the reaction is completed 50% in 15 minutes.
- MHT CET - 2024
- Chemistry
- kinetics equations
- IUPAC Name of Glyceraldehyde is:
- MHT CET - 2024
- Chemistry
- kinetics equations
- IUPAC Name of Acetone is:
- MHT CET - 2024
- Chemistry
- kinetics equations
- The half-life period of a first order reaction is 1000 seconds. Its rate constant is:
- KEAM - 2024
- Chemistry
- kinetics equations
View More Questions
Questions Asked in VITEEE exam
- Find the value of \( x \) in the following equation: \[ \frac{2}{x} + \frac{3}{x + 1} = 1 \]
- VITEEE - 2025
- Algebra
- How many numbers between 0 and 9 look the same when observed in a mirror?
- VITEEE - 2025
- Odd one Out
- In a code language, 'TIGER' is written as 'JUISF'. How will 'EQUAL' be written in that language?
- VITEEE - 2025
- Odd one Out
- In a code language, 'TIGER' is written as 'JUISF'. How will 'EQUAL' be written in that language?
- VITEEE - 2025
- Data Interpretation
- TUV : VYB :: PRA : ?
- VITEEE - 2025
- Odd one Out
View More Questions
Concepts Used:
Kinetics Equations
It is branch of physics that defines motion with respect to space and time is known as kinematics.
Inverse Kinematics: Inverse Kinematics do the reverse of kinematics.
There are four basic kinematics equations:
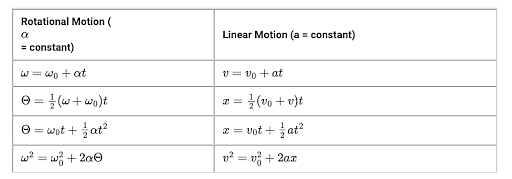
Rotational Kinematics Equations
Another branch of kinematics equations which deals with the rotational motion of anybody.