The correct sequence of reagents for the preparation of Q and R is:
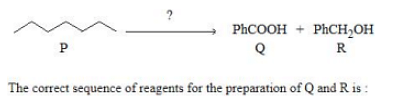
The correct sequence of reagents for the preparation of Q and R is:
(i) Cr2O3, 770K,20atm (ii) CrO2Cl2,H3O+; (iii) NaOH; (iv) H3O+
(i) Mo2O3,Δ; (ii) CrO2Cl2,H3O+; (iii) NaOH; (iv) H3O+
(i) CrO2Cl2,H3O+; (ii) Cr2O3,770K,20atm;(iii) NaOH; (iv) H3O+
(i) KMnO4,OH−; (ii) Mo2O3,Δ; (iii) NaOH; (iv) H3O+
The Correct Option is A
Approach Solution - 1

Approach Solution -2
1. Step 1: Oxidation of benzene to benzoquinone using CrO$_3$ at 770 K and 20 atm.
2. Step 2: Formation of phenol by further oxidation with CrO$_2$Cl$_2$ in acidic medium.
3. Step 3: Neutralization with NaOH to form phenoxide ion.
4. Step 4: Acidification with H$_3$O$^+$ to yield phenol. In the preparation of phenol from benzene, the use of CrO$_3$ and CrO$_2$Cl$_2$ ensures selective oxidation steps. Acidic and basic conditions aid in subsequent transformations.
Top Questions on Hydrocarbons
Which of the following hydrocarbons reacts easily with MeMgBr to give methane?

- WBJEE - 2026
- Chemistry
- Hydrocarbons
Consider the following reaction sequence.

- JEE Main - 2026
- Chemistry
- Hydrocarbons
- $\text{C}_3\text{H}_6\text{Cl}_2$ (X) $\xrightarrow[\Delta]{\text{NaNH}_2 (2 \text{eq})}$ (Y) $\xrightarrow[\text{(ii) NaBH}_4]{\text{(i) Hg(OAc)}_2/\text{H}_2\text{O}}$ $\text{CH}_3\text{COCH}_3$. (Y) $\xrightarrow[\Delta]{\text{Fe/tube}}$ (Z).
Statement-I : Y gives yellow ppt. with $\text{NaOH}/\text{I}_2$.
Statement-II : Two types of H-atoms and one aromatic ring is present in Z and ratio of Z and X is 1 : 3.
Choose the correct option.- JEE Main - 2026
- Chemistry
- Hydrocarbons
- Ph-CH=CH$_2 \xrightarrow[\text{(PhCOO)}_2]{\text{HBr}}$ Product.
Correct statement(s) regarding product :
(a) Ph-CH(Br)-CH$_3$ is minor product
(b) Benzene is also form a bi product
(c) Reaction follow free radical mechanism
(d) In absence of peroxide carbocation mechanism is followed- JEE Main - 2026
- Chemistry
- Hydrocarbons
- Correct stability order of alkene ::

- JEE Main - 2026
- Chemistry
- Hydrocarbons
Questions Asked in JEE Main exam
- The number of relations, defined on the set \{a, b, c, d\}, which are both reflexive and symmetric, is equal to:
- JEE Main - 2026
- Relations and Functions
- One mole of Cl$_2$(g) was passed into 2 L of cold 2 M KOH solution. After the reaction, the concentrations of Cl$^-$, ClO$^-$ and OH$^-$ are respectively (assume volume remains constant)
- JEE Main - 2026
- Chemical Reactions
- Let \(P\) be a point in the plane of the vectors \[ \vec{AB}=3\hat{i}+\hat{j}-\hat{k} \quad\text{and}\quad \vec{AC}=\hat{i}-\hat{j}+3\hat{k} \] such that \(P\) is equidistant from the lines \(AB\) and \(AC\). If \(|\vec{AP}|=\frac{\sqrt5}{2}\), then the area of triangle \(ABP\) is:
- JEE Main - 2026
- 3D Geometry
- The number of $3\times2$ matrices $A$, which can be formed using the elements of the set $\{-2,-1,0,1,2\}$ such that the sum of all the diagonal elements of $A^{T}A$ is $5$, is
- JEE Main - 2026
- Matrices
- Let \(P_1 : y = 4x^2\) and \(P_2 : y = x^2 + 27\) be two parabolas. If the area of the bounded region enclosed between \(P_1\) and \(P_2\) is six times the area of the bounded region enclosed between the line \(y = x\), the line \(x = 0\), and \(P_1\), then the required value is:
- JEE Main - 2026
- Conic sections
Concepts Used:
Hydrocarbons
Hydrocarbons can be described as organic compounds that consists only hydrogen and carbon atoms. These compounds are of different types and thereby have distinct natures. Hydrocarbons are colorless gases and are known for discharging faint odours. These have been categorized under four major classes named as alkynes, alkanes, alkenes, and aromatic hydrocarbons.
Types of Hydrocarbons
- Saturated hydrocarbons - Saturated hydrocarbons are those compounds where there is a single bond exists between carbon atoms and are saturated with atoms of hydrogen.
- Unsaturated hydrocarbons - Hydrocarbons comprises of at least one double or triple bond between carbon atoms are known as unsaturated hydrocarbons.
- Aliphatic hydrocarbons - The term denotes the hydrocarbons formed as an outcome of the chemical degradation of fats. Aliphatic hydrocarbons are basically chemical compounds.
- Aromatic hydrocarbons - They are distinguished because of the presence of benzene rings in them. They give away distinct types of aroma. These hydrocarbons comprises of only hydrogen and carbon atoms.