Name the reagents used in the following reactions:
(i)Oxidation of a primary alcohol to carboxylic acid.
(ii)Oxidation of a primary alcohol to aldehyde.
(iii)Bromination of phenol to 2,4,6-tribromophenol.
(iv)Benzyl alcohol to benzoic acid.
(v)Dehydration of propan-2-ol to propane.
(vi)Butan-2-one to butan-2-ol.
Name the reagents used in the following reactions:
(i)Oxidation of a primary alcohol to carboxylic acid.
(ii)Oxidation of a primary alcohol to aldehyde.
(iii)Bromination of phenol to 2,4,6-tribromophenol.
(iv)Benzyl alcohol to benzoic acid.
(v)Dehydration of propan-2-ol to propane.
(vi)Butan-2-one to butan-2-ol.
Solution and Explanation
(i)Acidified potassium permanganate

(ii)Pyridinium chlorochromate(PCC)
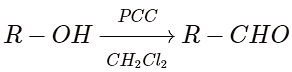
(iii) Bromine water

(iv)Acidified potassium permanganate

(v)85% phosphoric acid

(vi)NaBH4 or LiAlH4

Top Questions on Alcohols, Phenols And Ethers
- Can sodium ethoxide and t-butyl chloride be used for the preparation of t-butyl ethyl ether? Give suitable explanation. Justify your answer by suggesting the appropriate starting material required for preparation of t-butyl ethyl ether.
- CBSE CLASS XII - 2025
- Chemistry
- Alcohols, Phenols And Ethers
Arrange the following compounds in increasing order of their boiling points:

- CBSE CLASS XII - 2025
- Chemistry
- Alcohols, Phenols And Ethers
The reaction

suggests that phenol is :- CBSE CLASS XII - 2025
- Chemistry
- Alcohols, Phenols And Ethers
- Phenol reacts with Bromine (Br$_2$) water to form:
- CBSE CLASS XII - 2025
- Chemistry
- Alcohols, Phenols And Ethers
In the reaction R–OH + HCl $\xrightarrow{\text{ZnCl}_2}$ RCl + H$_2$O, what is the correct order of reactivity of alcohols?
- CBSE CLASS XII - 2025
- Chemistry
- Alcohols, Phenols And Ethers
Questions Asked in CBSE CLASS XII exam
- Write the cell reaction and calculate the e.m.f. of the following cell at 298 K:
\[ \text{Sn}(s) \mid \text{Sn}^{2+} (\text{0.004 M}) \parallel \text{H}^+ (\text{0.02 M}) \mid \text{H}_2 (\text{1 Bar}) \mid \text{Pt}(s) \]
(Given: \( E^\circ_{\text{Sn}^{2+}/\text{Sn}} = -0.14 \, \text{V}, E^\circ_{\text{H}^+/\text{H}_2} = 0.00 \, \text{V} \))- CBSE CLASS XII - 2025
- Electrochemistry
If vector \( \mathbf{a} = 3 \hat{i} + 2 \hat{j} - \hat{k} \) \text{ and } \( \mathbf{b} = \hat{i} - \hat{j} + \hat{k} \), then which of the following is correct?
- Find the value of $x$, if \[ \begin{bmatrix} 1 & 3 & 2 \\ 2 & 5 & 1 \\ 15 & 3 & 2 \end{bmatrix} \begin{bmatrix} 1 \\ x \\ 2 \end{bmatrix} = \begin{bmatrix} 0 \\ 0 \\ 0 \end{bmatrix} \]
- Two point charges of \( -5\,\mu C \) and \( 2\,\mu C \) are located in free space at \( (-4\,\text{cm}, 0) \) and \( (6\,\text{cm}, 0) \) respectively.
(a) Calculate the amount of work done to separate the two charges at infinite distance.
(b) If this system of charges was initially kept in an electric field \[ \vec{E} = \frac{A}{r^2}, \text{ where } A = 8 \times 10^4\, \text{N}\,\text{C}^{-1}\,\text{m}^2, \] calculate the electrostatic potential energy of the system.- CBSE CLASS XII - 2025
- Electrostatics
- 4,000 shares of ₹ 10 each were forfeited for non-payment of second and final call money of ₹ 2 per share. The minimum amount that the company must collect at the time of reissue of these shares will be :
- CBSE CLASS XII - 2025
- Accounting for Share Capital
Concepts Used:
Preparation - Alcohols, Phenols and Ethers
Alcohols, phenols, and ethers are organic compounds that can be prepared by various methods.
Preparation of Alcohols:
- Direct hydration of alkenes: Alcohols can be prepared by the addition of water to an alkene in the presence of a strong acid catalyst.
- Reduction of carbonyl compounds: Alcohols can be prepared by the reduction of aldehydes, ketones, or carboxylic acids using reducing agents like NaBH4 or LiAlH4.
- Grignard reaction: Alcohols can be prepared by reacting Grignard reagents with carbonyl compounds.
- Hydroboration-oxidation: Alcohols can be prepared by the hydroboration of alkenes followed by oxidation with an oxidizing agent like H2O2.
Preparation of Phenols:
- Hydrolysis of diazonium salts: Phenols can be prepared by the hydrolysis of diazonium salts, which are formed by the reaction of aniline with nitrous acid.
- Oxidation of sulfonic acids: Phenols can be prepared by the oxidation of sulfonic acids using strong oxidizing agents like potassium permanganate or chromic acid.
Preparation of Ethers:
- Williamson synthesis: Ethers can be prepared by the reaction of an alkoxide ion with a primary alkyl halide or tosylate in the presence of a strong base like NaOH or KOH.
- Dehydration of alcohols: Ethers can be prepared by the dehydration of alcohols in the presence of a strong acid catalyst like H2SO4.
In summary, alcohols, phenols, and ethers can be prepared by a variety of methods, including hydration, reduction, Grignard reaction, hydroboration-oxidation, hydrolysis, oxidation, Williamson synthesis, and dehydration. The choice of the method depends on the availability of starting materials, the desired product, and the conditions of the reaction.