Isoquinoline on treatment with oleum at 90°C yields majorly:
- Isoquinoline-3-sulfonic acid
- Isoquinoline-5-sulfonic acid
- Isoquinoline-6-sulfonic acid
- Isoquinoline-7-sulfonic acid
The Correct Option is B
Solution and Explanation
The reaction of isoquinoline with oleum at elevated temperatures is a sulfonation reaction. Isoquinoline, like quinoline, can undergo electrophilic aromatic substitution. The sulfonation of aromatic compounds usually involves the addition of a sulfonic acid group (\text{-SO_3H}) onto the aromatic ring.
In isoquinoline, the most reactive site for electrophilic substitution is determined by the electron density on different positions of the isoquinoline ring. The electrophile \text{SO}_3 can attack these positions to produce sulfonated derivatives.
The following factors influence the position of sulfonation in isoquinoline:
- Resonance Effect: The lone pairs on the nitrogen atom of isoquinoline can delocalize, affecting the electron density distribution in the ring.
- Inductive Effect: The nitrogen atom can exhibit an inductive effect, affecting electron density in the heterocycle.
Considering these effects, position 5 on the isoquinoline ring becomes one of the more favorable sites for electrophilic attack due to the balance between these effects. Therefore, the sulfonation primarily yields isoquinoline-5-sulfonic acid as the major product.
Thus, the correct answer to the question is:
- Isoquinoline-5-sulfonic acid
Top Questions on Heterocyclic Chemistry
- The acidity of the compounds shown below
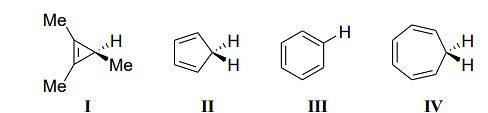
follows the order- IIT JAM CY - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy