The acidity of the compounds shown below
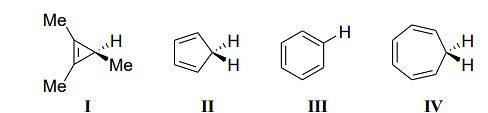
follows the order

follows the order
- II > I > III > IV
- II > IV > III > I
- I > II > IV > III
- III > IV > II > I
The Correct Option is B
Solution and Explanation
Compound I (the methyl group attached to a benzene ring with two hydrogen atoms attached to the other carbon atoms) has low acidity due to the electron-donating effects of the methyl group, which stabilize the negative charge on the conjugate base.
Compound II is more acidic because the electron-withdrawing substituent (the –NO2 group) helps stabilize the negative charge on the conjugate base by delocalizing it.
Compound III has a conjugate base that is stabilized by the aromatic ring, but it is still less acidic than II.
Compound IV is less acidic because the lack of any electron-withdrawing group makes it more difficult to stabilize the negative charge on the conjugate base.
Thus, the order of acidity follows \(II > IV > III > I,\) making (B) the correct answer.
Top Questions on Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- X and Y in the following reactions, respectively, are
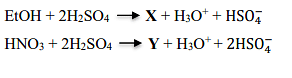
- IIT JAM CY - 2023
- Organic Chemistry
- Heterocyclic Chemistry
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry