In the reaction given below, the total number of atoms having sp2 hybridization in the major product P is ___.
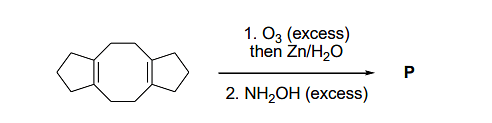
Correct Answer: 12
Solution and Explanation
The image depicts a reaction starting with a molecule (P) containing four carbon atoms (denoted by \( * \)), four nitrogen atoms, and four oxygen atoms. These atoms all exhibit sp2 hybridization. The steps include reactions with ozone (O3) followed by zinc and water (Zn/H2O), and then an excess of NH2OH (hydroxylamine).
Step 2: Reaction with Ozone
Initially, the molecule undergoes ozonolysis with excess ozone (O3), which typically cleaves double bonds. The reaction with ozone results in the formation of an intermediate with oxygen atoms inserted into the existing bonds, breaking the original carbon-carbon double bond.
Step 3: Zinc and Water Treatment
After ozonolysis, the product is treated with zinc (Zn) and water (H2O). Zinc is often used to reduce and remove oxygen groups that are attached to the carbon atoms. In this case, Zn/H2O helps reduce any oxygenated intermediates, likely leading to the formation of a more stable product.
Step 4: Reaction with Hydroxylamine (NH2OH)
Next, the molecule reacts with excess hydroxylamine (NH2OH), which typically reacts with carbonyl groups (as part of the process of forming oximes). The reaction results in the addition of NH2OH groups to the molecule at the sites where carbonyl groups were present, forming a structure with multiple NH2OH groups.
Step 5: Product Structure
The final product consists of four nitrogen atoms, four oxygen atoms, and four carbon atoms, all exhibiting sp2 hybridization. The resulting structure has four hydroxylamine groups (-NH2OH) attached to the carbon atoms, completing the product. The total atom count is 12: four carbon atoms, four nitrogen atoms, and four oxygen atoms.
Step 6: Conclusion
The reaction sequence shows how ozonolysis, followed by reduction and hydroxylamine addition, leads to a product with sp2 hybridized atoms. The final structure contains four nitrogen atoms, four oxygen atoms, and four carbon atoms, all exhibiting sp2 hybridization.
Product (P) contains four carbon atoms denoted by (*), along with four nitrogen and four oxygen atoms, totaling 12 atoms, all exhibiting sp2 hybridization.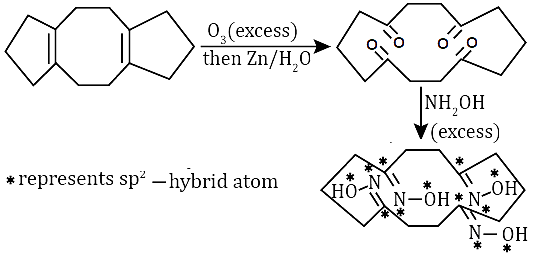
Top Questions on Hybridisation
Arrange the following in increasing order of solubility product:
\[ {Ca(OH)}_2, {AgBr}, {PbS}, {HgS} \]- JEE Main - 2025
- Chemistry
- Hybridisation
Concentrated nitric acid is labelled as 75% by mass. The volume in mL of the solution which contains 30 g of nitric acid is:
Given: Density of nitric acid solution is 1.25 g/mL.- JEE Main - 2025
- Chemistry
- Hybridisation
Match List - I with List - II.
List - I (Saccharides) List - II (Glycosidic linkages found)
(A) Sucrose (I) \( \alpha 1 - 4 \)
(B) Maltose (II) \( \alpha 1 - 4 \) and \( \alpha 1 - 6 \)
(C) Lactose (III) \( \alpha 1 - \beta 2 \)
(D) Amylopectin (IV) \( \beta 1 - 4 \)Choose the correct answer from the options given below:
- JEE Main - 2025
- Chemistry
- Hybridisation
Match List - I with List - II.
List - I (Complex) List - II (Hybridisation) (A) \([\text{CoF}_6]^{3-}\) (I) \( d^2 sp^3 \) (B) \([\text{NiCl}_4]^{2-}\) (II) \( sp^3 \) (C) \([\text{Co(NH}_3)_6]^{3+}\) (III) \( sp^3 d^2 \) (D) \([\text{Ni(CN}_4]^{2-}\) (IV) \( dsp^2 \)
Choose the correct answer from the options given below:- JEE Main - 2025
- Chemistry
- Hybridisation
- The change in hybridisation (if any) of the 'Al' atom in the following reaction is
AlCl3 + Cl- → AlCl4-
- KCET - 2025
- Chemistry
- Hybridisation
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties
Concepts Used:
Hybridisation
Hybridization refers to the concept of combining atomic orbitals in order to form new hybrid orbitals that are appropriate to represent their bonding properties. Hybridization influences the bond length and bond strength in organic compounds.
Types of Hybridization:
sp Hybridization
sp hybridization is observed while one s and one p orbital inside the identical principal shell of an atom mix to shape two new equal orbitals. The new orbitals formed are referred to as sp hybridized orbitals.
sp2 Hybridization
sp2 hybridization is observed whilst ones and p orbitals of the same shell of an atom blend to shape three equivalent orbitals. The new orbitals formed are referred to as sp2 hybrid orbitals.
sp3 Hybridization
When one ‘s’ orbital and 3 ‘p’ orbitals belonging to the identical shell of an atom blend together to shape 4 new equal orbitals, the sort of hybridization is referred to as a tetrahedral hybridization or sp3.
sp3d Hybridization
sp3d hybridization involves the joining of 3p orbitals and 1d orbital to form 5 sp3d hybridized orbitals of identical energy. They possess trigonal bipyramidal geometry.
sp3d2 Hybridization
With 1 s three p’s and two d’s, there is a formation of 6 new and identical sp3d2 orbitals.