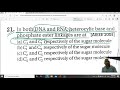
In both DNA and RNA, heterocyclic base and phosphate ester linkages are at
- (A) and respectively of the sugar molecule
- (B) and respectively of the sugar molecule
- (C) and respectively of the sugar molecule
- (D) and respectively of the sugar molecule
The Correct Option is B
Approach Solution - 1
In DNA and RNA heterocyclic base and phosphate ester are at and respectively of the sugar molecule.

Approach Solution -2
Phosphate ester linkage is the covalent bond that will connect the sugar molecules with the backbone. This linkage occurs between 3’ carbon of one sugar to the 5’ carbon of the adjacent carbon. The phosphate will provide a negative charge and maintain the stability.
On the other hand, the heterocyclic base is attached to the Carbon-1’ of both DNA and RNA sugar molecules. The bases present in them are adenine, guanine, cytosine, uracil and thymine.
Learn with videos:
Top Questions on Chemistry in Everyday Life
Given below are two statements:
Statement I: D-(+)-glucose + D-(+)-fructose $\xrightarrow{H_2O}$ sucrose sucrose $\xrightarrow{\text{Hydrolysis}}$ D-(+)-glucose + D-(+)-fructose
Statement II: Invert sugar is formed during sucrose hydrolysis.
In the light of the above statements, choose the correct answer from the options given below -- JEE Main - 2025
- Chemistry
- Chemistry in Everyday Life
- What is the pH of a 0.01 M solution of HCl?
- BITSAT - 2025
- Chemistry
- Chemistry in Everyday Life
- Arrange the following in the increasing order of oxidation number of nitrogen
A. N$_2$O
B. NO$_3^-$
C. NO
D. NO$_2$- TS EAMCET - 2025
- Chemistry
- Chemistry in Everyday Life
- Which of these separation technique pairs is correctly matched?
- AIIMS Paramedical - 2025
- Chemistry
- Chemistry in Everyday Life
- Which of the following is an example of antifertility drug?
- TS EAMCET - 2025
- Chemistry
- Chemistry in Everyday Life
Questions Asked in JEE Main exam
- The sum of all possible values of \( n \in \mathbb{N} \), so that the coefficients of \(x, x^2\) and \(x^3\) in the expansion of \((1+x^2)^2(1+x)^n\) are in arithmetic progression is :
- JEE Main - 2026
- Integration
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves