Question:
In a lactic acid solution at pH 4.8, the concentrations of lactic acid and lactate are 0.01 M and 0.087 M, respectively. The calculated pKa of lactic acid is _______. (Round off to one decimal place)
In a lactic acid solution at pH 4.8, the concentrations of lactic acid and lactate are 0.01 M and 0.087 M, respectively. The calculated pKa of lactic acid is _______. (Round off to one decimal place)
Show Hint
Always remember to use the Henderson-Hasselbalch equation for quick estimations of pKa from the pH and concentration ratios of conjugate acid-base pairs.
Updated On: Feb 2, 2025
Hide Solution
Verified By Collegedunia
Solution and Explanation
The relationship between the acid dissociation constant (\( K_a \)), the concentrations of the acid (\(\text{HA}\)) and its conjugate base (\(\text{A}^-\)), and the pH of the solution can be described using the Henderson-Hasselbalch equation:
\[
\text{pH} = \text{p}K_a + \log \left( \frac{[\text{A}^-]}{[\text{HA}]} \right)
\]
Given:
- \(\text{pH} = 4.8\)
- \([\text{HA}] = 0.01 \, M\) (concentration of lactic acid)
- \([\text{A}^-] = 0.087 \, M\) (concentration of lactate)
Rounding off to one decimal place, the \( \text{p}K_a \) of lactic acid is:
\[ \text{p}K_a = 3.9 \] Conclusion:This calculation demonstrates how the dissociation constant for lactic acid can be deduced from the equilibrium concentrations of the acid and its conjugate base at a specific pH.
Was this answer helpful?
0
0
Top Questions on Nucleic acid: replication, transcription, splicing, translation
- Calculate the λmax of the following molecule:
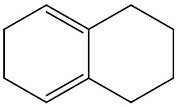
- GPAT - 2024
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- Calculate the \( \lambda_{\text{max}} \) of the following molecule:
- GPAT - 2024
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- DNA and RNA contain the following two major purine bases:
- GPAT - 2023
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- Given below are two statements
Statement I: Sequencing of DNA is much easier than RNA sequencing due to greater stability
Statement II: The chemical method of DNA sequencing (Maxam & Gilbert) works for only single stranded DNA
In light of the above statements, choose the correct answer from the options given below- GPAT - 2022
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- The enzyme that transcribes the eukaryotic genes encoding precursor ribosomal RNAs (pre-rRNAs) of 28S, 18S and 5.8S rRNAs is
- GATE BT - 2021
- Genetics, Cellular and Molecular Biology
- Nucleic acid: replication, transcription, splicing, translation
View More Questions
Questions Asked in GATE XL exam
- Imagine a population of diploid species in Hardy-Weinberg equilibrium. The population has two alleles for a gene which are ‘a’ and ‘A’. The number of individuals with ‘aa’ genotype in this population is 1 in 10000. The frequency of the allele ‘A’ in the population is ............ (up to two decimal places)
- GATE XL - 2025
- Zoology
- The \( F_{121} \) value of a known microorganism with \( Z \) value of \( 11^\circ C \) is 2.4 min for 99.9999% inactivation. For a 12D inactivation of the said microorganism at \( 143^\circ C \), the \( F \) value (in min) is .......... {(rounded off to 3 decimal places)}
- GATE XL - 2025
- Food Technology
- In a typical grinding operation, 80% of the feed material passes through a sieve opening of 4.75 mm; whereas, 80% of the ground product passes through 0.5 mm opening. If the power required to grind 2 tonnes/h of the feed material is 3.8 kW, the work index of the material is ........ (rounded off to 2 decimal places)
- GATE XL - 2025
- Food Technology
- The true density and bulk density of wheat grains are 1280 kg/m\(^3\) and 740 kg/m\(^3\), respectively. The porosity of the grains is ......... (rounded off to 2 decimal places).
- GATE XL - 2025
- Food Technology
- An enzyme, which follows Michaelis-Menten equation, catalyzes the reaction A\(\rightarrow\)B. When enzyme and substrate concentrations are 15 nM and 10 \(\mu\)M, respectively, the reaction velocity is 5 \(\mu\)M s\(^{-1}\). If \(K_m\) for the substrate A is 5 \(\mu\)M, the kinetic efficiency of the enzyme will be ______ \(\times 10^6\) M\(^{-1}\) s\(^{-1}\) (in integer).
- GATE XL - 2025
- Zoology
View More Questions