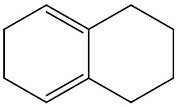
Calculate the \( \lambda_{\text{max}} \) of the following molecule:
Show Hint
- 283 nm
- 273 nm
- 234 nm
- 244 nm
The Correct Option is B
Solution and Explanation
The \( \lambda_{\text{max}} \), or the maximum absorption wavelength, is an important characteristic of a molecule and is typically determined by its conjugated system, i.e., the extent of \(\pi-electron \) delocalization. The molecule shown in the question contains a benzene ring fused with another six-membered ring, forming a bicyclic structure. In general, the \(\lambda _\text{max}\) of such molecules can be predicted based on their conjugation. The molecule in question has an extended conjugated system, and based on similar structures and their known absorption maxima, we expect the \(\lambda _\text{max}\) for this molecule to be around 273 nm. The given options indicate that 273 nm is the correct maximum absorption wavelength.
Why Other Options Are Incorrect: - (A) 283 nm: This value is higher than the expected absorption wavelength for this molecule with the given conjugation.
- (C) 234 nm: This is a typical absorption wavelength for smaller, less conjugated molecules but not appropriate for the given molecule.
- (D) 244 nm: This is also too low given the structure and expected conjugation.
Thus, the correct value for \( \lambda_{\text{max}} \) is 273 nm, corresponding to option (B).
Top Questions on Nucleic acid: replication, transcription, splicing, translation
- Calculate the λmax of the following molecule:
- GPAT - 2024
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- In a lactic acid solution at pH 4.8, the concentrations of lactic acid and lactate are 0.01 M and 0.087 M, respectively. The calculated pKa of lactic acid is _______. (Round off to one decimal place)
- GATE XL - 2024
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- DNA and RNA contain the following two major purine bases:
- GPAT - 2023
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- Given below are two statements
Statement I: Sequencing of DNA is much easier than RNA sequencing due to greater stability
Statement II: The chemical method of DNA sequencing (Maxam & Gilbert) works for only single stranded DNA
In light of the above statements, choose the correct answer from the options given below- GPAT - 2022
- Biochemistry
- Nucleic acid: replication, transcription, splicing, translation
- The enzyme that transcribes the eukaryotic genes encoding precursor ribosomal RNAs (pre-rRNAs) of 28S, 18S and 5.8S rRNAs is
- GATE BT - 2021
- Genetics, Cellular and Molecular Biology
- Nucleic acid: replication, transcription, splicing, translation
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy